Shifting the Paradigm in HIV Prevention and Treatment Service Delivery Toward Differentiated Care for Youth
ABSTRACT | Despite significant progress in the fight against HIV/AIDS in the United States, HIV prevention and treatment disparities among key populations remain a national public health concern. While new HIV diagnoses are increasing among people under age 30—in particular among racial, ethnic, and sexual minority adolescents and young adults (AYA)—dominant prevention and treatment paradigms too often inadequately consider the unique HIV service needs of AYA. To address this gap, we characterize persistent and largely overlooked AYA disparities across the HIV prevention and treatment continuum, identify AYA-specific limitations in extant resources for improving HIV service delivery in the United States, and propose a novel AYA-centered differentiated care framework adapted to the unique ecological and developmental factors shaping engagement, adherence, and retention in HIV services among AYA. Shifting the paradigm for AYA to differentiated HIV care is a promising approach that warrants implementation and evaluation as part of reinforced national efforts to end the HIV epidemic in the United States by 2030.
Introduction
Today, more than 125 million people in the United States are under age 30, representing nearly 40 percent of the overall population [1]. Projections suggest that the number of adolescents and young adults (AYA) in the United States will grow consistently over the foreseeable future, largely driven by increases in racial and ethnic minority populations. By 2030, minority AYA will account for more than half the United States population under 30 [2]. AYA, particularly those from racial and ethnic minorities, are disproportionately affected by sexual and reproductive health (SRH) disparities, including unintended pregnancy, sexually transmitted infections (STIs), and HIV [3,4,5]. In recent years, new HIV diagnoses among AYA have increased significantly [6]. However, dominant prevention and treatment paradigms too often inadequately consider the unique HIV service needs of AYA. To address this gap, we characterize persistent and largely overlooked AYA disparities across the HIV prevention and treatment continuum, identify AYA-specific limitations in extant resources for improving HIV service delivery in the United States, and propose a novel AYA-centered differentiated care framework adapted to the unique ecological and developmental factors shaping engagement, adherence, and retention in HIV services among AYA.
The Invisible Crisis: Neglected AYA
Since 2012, the incidence of HIV diagnoses among AYA has increased significantly [6]. However, the accelerating HIV/AIDS epidemic among AYA has gone largely unrecognized, overshadowed by aggregate data reflecting overall progress among adult individuals newly diagnosed with HIV (see Figure 1) [6]. Today, AYA aged 13 to 29 account for two in every five new HIV diagnoses, and the number of AYA living with diagnosed HIV increased for the fourth consecutive year in 2016 [6]. Among AYA, those from racial, ethnic, and sexual minorities account for the majority of HIV diagnoses. For example, 79 percent of AYA aged 13 to 29 who are newly diagnosed with HIV are from racial and ethnic minority groups, and 79 percent are young men who have sex with men (YMSM) [6]. Since 2012, the annual number of new HIV diagnoses increased by 17 percent among Latino YMSM and by 9 percent among Black YMSM [6].
AYA who are at risk of, or living with, HIV are engaged and retained in HIV services at rates lower than those of their older counterparts. Indeed, there are disparities among age groups across the HIV care continuum (see Figure 2) [7]. A 2018 National Health Statistics Report shows that 74 percent of men and 64 percent of women aged 15 to 24 have never been tested for HIV [8]. The Centers for Disease Control and Prevention (CDC) estimates that, as a result, about half of AYA aged 13 to 24 living with HIV in the United States—the highest rate of undiagnosed HIV among all age groups—are unaware of their infection [9]. Of AYA diagnosed with HIV, approximately 75 percent receive HIV care; however, a mere 56 percent are retained in care services [10]. Consequently, about one in two AYA who are living with HIV and are aware of their status do not achieve viral suppression [10]. It is important to note that youth who have been diagnosed but do not achieve viral suppression, along with their peers living with HIV who remain undiagnosed, are more likely to transmit the infection to uninfected sex and substance-using partners [11].
Recent biomedical innovations have significantly expanded HIV prevention options. Numerous studies affirm the efficacy of pre- and post-exposure prophylaxis (PrEP/PEP) [12–17]. PrEP efficacy for preventing HIV acquisition among at-risk individuals is over 90 percent when taken as prescribed [12]. Two AYA-specific PrEP studies confirm the efficacy of PrEP for AYA. However, sub-optimal retention and adherence in both studies resulted in seroconversion in individuals with PrEP concentrations below therapeutic levels [13,18].
In addition, there are concerns about behavioral adaptation and disinhibition among PrEP users [19,20]. AYA PrEP users are particularly vulnerable to HIV and STI infections when reductions in condom use (i.e., behavioral adaptation) and increased risk behavior (i.e., behavioral disinhibition) occur, given sub-optimal adherence. Although 700,000 AYA who are at risk of HIV infection are estimated to have indications for PrEP in the United States [21], AYA aged 13 to 24 represented merely 12 percent of at least 100,000 PrEP users in 2017 [22].
Against the Backdrop of Progress
The continuing US HIV crisis affecting AYA has emerged against the backdrop of decadelong population-level progress in the fight against HIV. Most recently, the federal government has revealed a national strategic plan to leverage existing public health tools and resources to completely end HIV transmission in the United States by 2030 [23-25]. However, rising numbers of HIV diagnoses among AYA jeopardize the attainment of goals outlined in the US Department of Health and Human Services’ (HHS) Ending the HIV Epidemic: A Plan for America strategy [23-25]. Given the planned and reinforced public health efforts to halt HIV transmission in the United States, it is time to turn national attention to those left behind in the fight against HIV/AIDS—AYA who are at the greatest risk of, or living with, HIV. Novel delivery approaches are sorely needed to address the unique developmental and contextual barriers for engaging and retaining AYA in HIV prevention and treatment services.
Resources for Improving HIV Services in the United States
In the United States, efforts to improve HIV service delivery to key populations largely rely on guidelines and resources provided by the HHS. The HHS maintains clinical guidelines for the delivery of antiretroviral therapy (ART), PEP, PrEP, and other HIV services [26]. In addition, the CDC maintains the Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention, which includes chapters addressing linkage to, retention in, and re-engagement in HIV care, as well as medication adherence [27]. Here, we review the evidence-based interventions (EBIs) and their associated peer-review publications listed in the CDC compendium as of January 2019. The interventions included in our review have been evaluated in randomized clinical trials with efficacy and methodological rigor, as designated by the CDC. In the subsequent sections, we synthesize existing evidence in the CDC compendium for EBIs at each stage of the HIV care continuum. Table 1 provides a detailed analysis of the components used in the EBIs and endorsed by the CDC, including HIV service type, service frequency and intensity, HIV service provider, and the setting and means of service delivery.
Linkage to Care
The CDC compendium includes three effective EBIs supporting the linkage to care of newly diagnosed people living with HIV [27]. For the purposes of the compendium, the operational definition of “linkage to care” is the first completed medical visit within six months of HIV diagnosis, as shown in medical, administrative, or agency records or in surveillance reports. It is important to note, however, that current guidelines recommend linkage to care as soon as possible within 30 days of HIV diagnosis [26]. Only one of the three EBIs was evaluated in the United States, and none were designed or evaluated specifically for AYA populations living with HIV (under 30 years old). Two of the three EBIs rely primarily on in-person counseling, while the other emphasizes individualized case management. Interestingly, all three linkage-to-care interventions share a common set of components. Linkage-to-care EBIs rely on meeting newly diagnosed clients on at least a monthly basis and offering appointments outside the clinical setting. While all three linkage-to-care EBIs rely on social service providers for intervention delivery, one intervention also involves allied health professionals specifically trained in post-diagnosis counseling.
Retention in Care
The CDC compendium includes four effective EBIs for improving retention in HIV care [27]. The operational definition of “retention in care” refers to consecutive medical visits within six months, as shown in medical, administrative, or agency records or in surveillance reports. Three out of the four retention-in-care interventions have been evaluated primarily for individuals from racial and ethnic minorities, and one EBI was designed and evaluated specifically for opioid substance users living with HIV. However, none of the EBIs were designed or specifically evaluated for AYA populations (under 30 years old). Interestingly, the retention-in-care interventions included in the CDC compendium have few commonalities. While all four EBIs are delivered at least partly in the clinical setting, the interventions differ across the types of services delivered and the frequency of client contact. While two interventions involve health care providers and allied health professionals, the remaining two are delivered by social service providers.
Medication Adherence
The CDC compendium includes 13 effective EBIs addressing medication adherence among people living with HIV [27]. “Efficacy in the improvement of medication adherence” is operationally defined as a combination of at least one improved behavioral adherence outcome—as assessed by the Medication Event Monitoring System (MEMS©) caps, pill counts, pharmacy refills, or self-reported medication adherence—and improved HIV viral load. The majority of interventions were evaluated primarily for individuals from racial and ethnic minority groups, and two EBIs were designed and evaluated with a specific population focus, namely for substance users living with HIV and couples with HIV serodiscordant status. Many recurring themes emerge. The majority of effective adherence interventions (eight out of 13) rely on individual-, group-, and partner-based counseling. In all cases, counseling is combined with a focus on identification of individual adherence barriers and facilitators, on HIV-related educational activities, or both. Roughly half of the interventions include at least weekly contact with clients, and three of those EBIs include daily reminders. Nine interventions are delivered partly or exclusively in the clinical setting, while the remaining four are delivered exclusively in nonclinical settings, including electronically and by telephone. The majority of EBIs are delivered by social service providers. While 12 out of 13 EBIs were not designed and evaluated specifically for AYA, one effective adherence intervention targets AYA aged 16 to 29. The only AYA-focused intervention, TXTXT, relies on daily adherence reminders delivered by text message over the course of six months [28].
[a] Muhamadi, L., N. M. Tumwesigye, D. Kadobera, G. Marrone, F. Wabwire-Mangen, G. Pariyo, S. Peterson, and A. M. Ekstrom. 2011. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre-antiretroviral care in eastern Uganda. Trials 12:184.1-184.11. https://dx.doi.org/10.1186%2F1745-6215-12-184 [b] Ruzagira, E., H. Grosskurth, A. Kamali, and K. Baisley. 2017. Brief counselling after home-based HIV counselling and testing strongly increases linkage to care: A cluster-randomized trial in Uganda. Journal of the International AIDS Society 20(2):e25014. https://doi.org/10.1002/jia2.25014 [c] Ruzagira, E., K. Baisley, A. Kamali, and H. Grosskurth. 2017. An open-label cluster randomised trial to evaluate the effectiveness of a counselling intervention on linkage to care among HIV-infected patients in Uganda: Study design. Contemporary Clinical Trials Communications 5:56-62. https://doi.org/10.1016/j.conctc.2016.12.003 [d] Gardner, L. I., L. R. Metsch, P. Anderson-Mahoney, A. M. Loughlin, C. del Rio, S. Strathdee, S. L. Sansom, H. A. Siegal, A. E. Greenberg, S. D. Holmberg, and the Antiretroviral Treatment and Access Study Group. 2005. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 19(4):423-431 [e] Craw, J. A., L. I. Gardner, G. Marks, R. C. Rapp, J. Bosshart, W. A. Duffus, A. Rossman, S. L. Coughlin, D. Gruber, L. A. Safford, J. Overton, and K. Schmitt. 2008. Brief strengths-based case management promotes entry into HIV medical care: Results of the Anti-Retroviral Treatment Access Study-II. Journal of Acquired Immune Deficiency Syndromes 47(5):597-606. https://doi.org/10.1097/QAI.0b013e3181684c51 [f] Lucas, G. M., A. Chaudhry, J. Hsu, T. Woodson, B. Lau, Y. Olsen, J. C. Keruly, D. A. Fiellin, R. Finkelstein, P. Barditch-Crovo, K. Cook, and R. D. Moore. 2010. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Annals of Internal Medicine 152(11):704-711. https://doi.org/10.7326/0003-4819-152-11-201006010-00003 [g] Gardner, L. I., T. P. Giordano, G. Marks, T. E. Wilson, J. A. Craw, M. L. Drainoni, J. C. Keruly, A. E. Rodriguez, F. Malitz, R. D. Moore, L. A. Bradley-Springer, S. Holman, C. E. Rose, S. Girde, M. Sullivan, L. R. Metsch, M. Saag, M. J. Mugavero, and the Retention-in-Care Study Group. 2014. Enhanced personal contact with HIV patients improves retention in primary care: A randomized trial in six US HIV clinics. Clinical Infectious Diseases 59(5):725-734. https://doi.org/10.1093/cid/ciu357 [h] Robbins, G. K., W. Lester, K. L. Johnson, Y. Chang, G. Estey, D. Surrao, K. Zachary, S. M. Lammert, H. C. Chueh, J. B. Meigs, and K. A. Freedberg. 2012. Efficacy of a clinical decision-support system in an HIV practice: A randomized trial. Annals of Internal Medicine 157(11)757-766. doi:10.7326/0003-4819-157-11-201212040-00003 [i] Williams, A. B., K. P. Fennie, C. A. Bova, J. D. Burgess, K. A. Danvers, and K. D. Dieckhaus. 2006. Home visits to improve adherence to highly active antiretroviral therapy: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes 42(3):314-321. https://doi.org/10.1097/01.qai.0000221681.60187.88 [j] Kurth, A. E., F. Spielberg, C. M. Cleland, B. Lambdin, D. R. Bangsberg, P. A. Frick, A. O. Severynen, M. Clausen, R. G. Norman, D. Lockhart, J. M. Simoni, and K. K. Holmes. 2014. Computerized counseling reduces HIV-1 viral load and sexual transmission risk: Findings from a randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes 65(5):611-620. https://dx.doi.org/10.1097%2FQAI.0000000000000100 [k] Altice, F. L., D. S. Maru, R. D. Bruce, S. A. Springer, and G. H. Friedland. 2007. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: A prospective, randomized, controlled trial. Clinical Infectious Diseases 45(6):770-778. https://doi.org/10.1086/521166 [l] Healthy Living Project Team. 2007. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: The Healthy Living Project randomized controlled study. Journal of Acquired Immune Deficiency Syndromes 44(2):213-221. https://doi.org/10.1097/QAI.0b013e31802c0cae [m] Johnson, M. O., E. Charlebois, S. F. Morin, R. H. Remien, M. A. Chesney, and National Institute of Mental Health Healthy Living Project Team. 2007. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: The Healthy Living Project randomized controlled study. Journal of Acquired Immune Deficiency Syndromes 46(5):574-580. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2442469 [n] Koenig, L. J., S. L. Pals, T. Bush, M. Pratt Palmore, D. Stratford, and T. V. Ellerbrock. 2008. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychology 27(2):159-169. https://doi.org/10.1037/0278-6133.27.2.159 [o] Kalichman, S. C., C. Cherry, M. O. Kalichman, C. M. Amaral, D. White, H. Pope, C. Swetzes, L. Eaton, R. Macy, and D. Cain. 2011. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. American Journal of Public Health 101(3):531-538. https://dx.doi.org/10.2105%2FAJPH.2010.197608 [p] Gross, R., S. L. Bellamy, J. Chapman, X. Han, J. O’Dour, S. C. Palmer, P. S. Houts, J. C. Coyne, and B. L. Strom. 2013. Managed problem solving for antiretroviral therapy adherence: A randomized trial. JAMA Internal Medicine 173(4):300-306. https://doi.org/10.1001/jamainternmed.2013.2152 [q] Simoni, J. M., D. Huh, P. A. Frick, C. R. Pearson, M. P. Andrasik, P. J. Dunbar, and T. M. Hooton. 2009. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes 52(4):465-473. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2795576 [r] Milam, J., J. L. Richardson, A. McCutchan, S. Stoyanoff, J. Weiss, C. Kemper, R. A. Larsen, H. Hollander, P. Weissmuller, and R. Bolan. 2005. Effect of a brief antiretroviral adherence intervention delivered by HIV care providers. Journal of Acquired Immune Deficiency Syndromes 40(3):356-363 [s] Kalichman, S. C., M. O. Kalichman, C. Cherry, L. A. Eaton, D. Cruess, and R. F. Schinazi. 2016. Randomized factorial trial of phone-delivered support counseling and daily text message reminders for HIV treatment adherence. Journal of Acquired Immune Deficiency Syndrome 73(1):47-54. https://doi.org/10.1097/QAI.0000000000001020 [t] Remien, R. H., M. J. Stirratt, C. Dolezal, J. S. Dognin, G. J. Wagner, A. Carballo-Dieguez, N. El-Bassel, and T. M. Jung. 2005. Couple-focused support to improve HIV medication adherence: A randomized controlled trial. AIDS 19(8):807-814 [u] Garofalo, R., L. M. Kuhns, A. Hotton, A. Johnson, A. Muldoon, and D. Rice. 2016. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS and Behavior 20(5):1049-1059. https://dx.doi.org/10.1007%2Fs10461-015-1192-x.
Misalignment between Existing EBIs and the HIV Care Continuum for AYA
Current EBIs endorsed by the CDC do not adequately address AYA-specific HIV prevention and treatment needs. The EBIs included in the compendium overwhelmingly focus on adult populations and seldom address the unique developmental and contextual factors shaping AYA engagement, adherence, and retention in HIV services. Only one EBI in the compendium was developed and evaluated for AYAs in particular. Interestingly, the type of service, frequency of patient contact, HIV service provider, and means and setting of service delivery used by the only AYA-centered EBI differ markedly from those used by the majority of the adult-focused EBIs. TXTXT relies on adherence reminders, daily patient contact, and automated service delivery via text message. By contrast, the most common intervention components in adult-focused interventions include counseling with individualized assessments of the barriers and facilitators of linkage, retention, and adherence and/or HIV-related educational activities; at least monthly patient contact; service delivery by social service providers; and service delivery primarily in the clinical setting. Furthermore, the largest declines in the HIV care continuum for AYA are in engagement and retention in HIV care. One in every four AYA living with diagnosed HIV is not receiving care, and one in four AYA who receive care is not retained in care [10]. However, the majority of EBIs in the compendium (13 out of 19), including the only AYA-specific intervention, focus on supporting medication adherence, rather than linkage and retention in care (see Figure 3) [10,27].
Additional Resources
In addition to CDC-endorsed EBIs, the HHS Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV serves as a resource for improving HIV service delivery [29]. The HHS provides recommendations regarding three important themes of HIV care delivery to AYA, specifically medication adherence, comprehensive SRH services, and transition into adult HIV care [29]. The guidelines highlight AYA-specific factors contributing to difficulties with medication adherence that warrant individualized clinical responses. Recommendations include implementing adherence support systems, such as reminder systems; avoiding complex medication regimens, and directly observing therapy. For severe or recurring adherence problems, regimens involving high resistance barriers and delay or suspension of antiretroviral therapy may be considered. In addition, the HHS recommends incorporating comprehensive SRH services into HIV care for AYA, including regular screening for STIs. Furthermore, the guidelines emphasize the importance of facilitating the transition into adult care for AYA living with HIV. Disengagement from HIV care among AYA often coincides with the transition from AYA-specific HIV care services to adult clinics. The HHS recommendations for transition care include pre-transition interventions, such as individualized transition plans, as well as post-transition interventions, such as support groups. While the HHS recommendations represent best practices in HIV care delivery to AYA, few have been rigorously evaluated within randomized clinical efficacy trials.
The Adolescent Medicine Trials Network and HRSA Building Futures Toolkit
In the United States, the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) has led efforts to explore targeted strategies for improving HIV prevention and care continuum outcomes among AYA [30]. Since 2001, the ATN has initiated roughly a hundred scientific studies, significantly advancing the understanding of the underlying factors driving HIV disparities among AYA. In addition, ATN research has expanded the HIV prevention and treatment options available to AYA. Based on ATN studies 110 and 113, the US Food and Drug Administration approved Truvada as PrEP for minors in 2018 [13,18]. Furthermore, Project ACCEPT, a group-based linkage-to-care intervention for AYA, successfully improved engagement in care, ART uptake, and viral loads among AYA in a small-scale randomized clinical trial, and additional AYA-focused interventions are currently in development or under evaluation [31,32].
In 2018, the US Health Resources and Services Administration’s (HRSA) HIV/AIDS Bureau released the Building Futures: Supporting Youth Living with HIV Technical Assistance Toolkit, containing AYA-specific best practices identified by Ryan White HIV/AIDS Program-funded HIV service providers [33]. The toolkit serves as a resource to support AYA-friendly clinic infrastructure, staffing, and services for HIV prevention and treatment. Important best practices that emerge from the toolkit include frequent and informal communication with AYA, such as text and social media; provision of support services beyond medical care by interdisciplinary staff (e.g., AYA support groups; mental health and substance abuse services; and assistance with housing, transportation, and job training); an AYA- and LGBTQ-friendly and culturally appropriate service environment (e.g., confidentiality and privacy; inclusive language; inviting physical environment; and sensitivity to cultural background, sexual orientation, and gender identity); and proactive retention and re-engagement efforts targeting AYA who are at risk or have dropped out of care. Furthermore, the toolkit encourages active AYA involvement in the development of HIV prevention and treatment programs.
While the existing CDC compendium and HHS HIV/AIDS treatment guidelines, coupled with the work of the ATN and HRSA’s HIV/AIDS Bureau, have improved the national capacity to fight the HIV epidemic among AYA, renewed research and program-based efforts to develop novel HIV service delivery approaches for AYA are sorely needed, given persistent HIV prevention and treatment disparities among key youth populations in the United States, including AYA from racial and ethnic minorities, transgender individuals, and YMSM.
Shifting the Paradigm of HIV Service Delivery for AYA: Differentiated Care
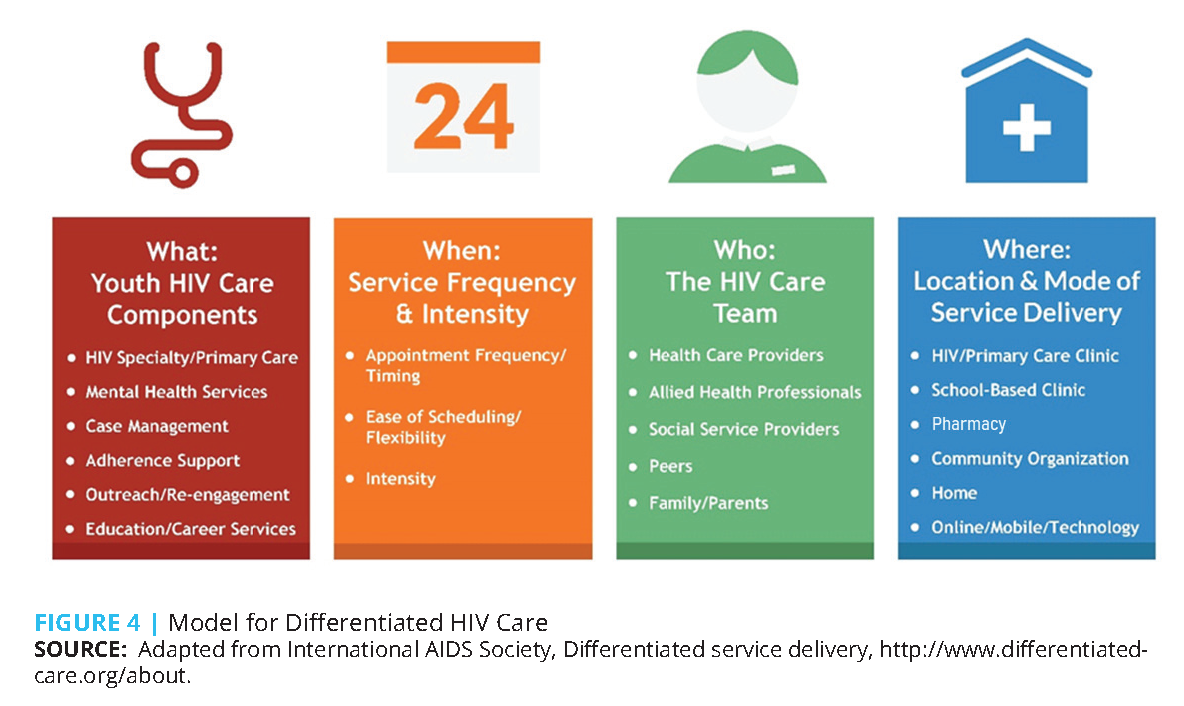
Globally, differentiated care approaches are widely used for HIV service delivery to key populations in resource-limited settings [34]. As opposed to a “one size fits all” approach, differentiated care tailors HIV service delivery across the prevention and care continuum to meet the needs, preferences, and expectations of key populations and hard-to-reach individuals [35,36]. By individualizing HIV care in terms of what kind of services are delivered, when those services are delivered, who delivers services, and where services are delivered (see Figure 4) [34], health care resources that are not used by stable patients become available for allocation to those requiring more intensive services [36]. Differentiated service delivery has been successful in improving HIV prevention, testing, and treatment outcomes, even for AYA [37-39]. Surprisingly, few efforts have been made to implement differentiated care models for AYA in the United States.
Future Directions for AYA-Centered HIV Prevention and Treatment
In the United States, the greatest need for effective HIV prevention and treatment services among AYA often exists in contexts of disadvantage and unequal opportunity, particularly among AYA from racial and ethnic minority groups, transgender individuals, and YMSM [40]. Experiences of stigmatization, homophobia and transphobia, and racial and ethnic discrimination during an important period of identity development can exacerbate the psychological trauma that is associated with HIV risk and diagnosis for AYA. However, existing HIV service delivery models too often do not adequately address the unique needs of AYA who are at the greatest risk of, or living with, HIV. We discuss an AYA-centered differentiated care framework adapted to the unique ecological and developmental factors shaping engagement, retention, and medication adherence in HIV services among AYA. We highlight considerations for four dimensions of differentiated service delivery—what, when, who, and where.
The current AYA-centered differentiated framework for HIV service delivery relies on timely adjustments to the care intensity and focus in response to changes in individual care outcomes. Given the significant variability in HIV prevention and treatment outcomes among AYA over time, the range of services that adequately address individual needs (what) may increase or decrease as treatment outcomes become more stable or unstable. Similarly, the appropriate frequency and intensity of HIV services (when) are dependent on individual progress toward the desired clinical outcomes, namely sustained viral suppression and quality of life. For example, HIV services for AYA that successfully sustain viral suppression may be limited to guideline-based standard AYA HIV care with moderate service intensity and frequency. On the other hand, AYA living with HIV who experience fluctuating viral loads, have not yet achieved viral suppression, or miss clinic appointments require more frequent and intensive services with distinct care foci. Careful and ongoing monitoring of AYA care outcomes provides the opportunity to develop and adjust individual care plans that combine care foci on standard AYA HIV care, adherence support, removal of barriers to treatment initiation, and engagement or re-engagement, as needed.
In turn, the elements included in individual care plans, and service intensity, inform the composition of the HIV care team (who). While health care providers play an active and important role in delivering medical care to all AYA who receive HIV services, allied health professionals, social service providers, and social support networks complement the HIV care team by providing additional components of individualized care, in particular to AYA requiring frequent and intensive support in achieving desired care outcomes. Furthermore, aligning individualized care plans with the best-suited settings and means of HIV service delivery (where) represents an opportunity to improve the effectiveness and efficiency of AYA-centered differentiated HIV care. Depending on the foci, intensity, and providers of individualized care plans, delivery of individual-, partner-, or group-based services in clinical or community settings, in the home, at ancillary services, or through online or mobile communication may represent the most effective support for AYA care outcomes.
For the purposes of the current differentiated care framework, we distinguish AYA at risk of, or living with, HIV at four levels of HIV care outcomes, with inherently different needs regarding HIV services. While the HIV prevention and treatment continua have primarily been regarded as separate frameworks, a growing body of literature integrates HIV prevention and treatment into one continuum [41-43]. Given the important role of both HIV treatment as prevention and HIV prevention in reducing the need for treatment, we also adopt an integrated approach to AYA-centered differentiated prevention and treatment services (see Figure 5).
I. (a) AYA who are at risk and sustain therapeutic levels of PrEP. HIV care plans for AYA who achieve and sustain therapeutic levels of PrEP focus on guideline-based PrEP service delivery with limited service frequency and intensity. Guideline recommendations for the provision of PrEP include follow-up visits every three months, repeated HIV screening, prescription refills, and assessments of continued adherence and risk status [44]. In addition, at least semi-annual STI screening is recommended [44]. It is important to note that PrEP services for youth should be as seamless and client-friendly as possible in order to decrease barriers to PrEP continuation. Emerging approaches that streamline the delivery of PrEP services and warrant further exploration include online application-based and home-based models [45-47]. These models allow for essential services—such as laboratory assessments, provider communication, and prescription refills—to be completed without in-person clinic visits [45].
(b) AYA living with HIV who sustain viral suppression. HIV care plans for AYA who achieve and sustain viral suppression focus on delivering the standard of care outlined in the HHS treatment guidelines [6]. In addition, promising AYA-centered approaches to HIV care provide an AYA-friendly environment, emphasize confidentiality and privacy, engage family members, and integrate HIV specialty care with primary, SRH, substance use, and mental health care [48-50]. Furthermore, it is important that all AYA who receive HIV treatment services are provided with adequate guidance prior to and during transition into adult HIV services [29]. Holistic approaches to HIV service provision for AYA that support healthy identity development, future aspirations and orientations, and well-being within a framework of overall wellness should be explored further [51-53].
II. (a) AYA who are at risk and who are on PrEP but are non-adherent. PrEP non-adherent AYA may require more frequent follow-up appointments and more intensive prevention services [18,45]. In addition, mobile adherence support has shown effectiveness in improving PrEP adherence among AYA in the United States [54]. To improve PrEP adherence among AYA, research should explore the potential of alternative PrEP dosing schedules that include intermittent or “on-demand” PrEP use before and after unprotected sex, in addition to long-acting agents for HIV prevention in various stages of development [55,56]. Furthermore, future research should explore approaches that leverage the familial context of AYA who are at risk, by providing interventions to support PrEP adherence.
(b) AYA living with HIV, with initial or fluctuating viral suppression, who are receiving HIV care. In addition to the standard of care outlined in existing HHS treatment guidelines, many AYA require targeted adherence support to achieve and sustain viral suppression. Adherence support services may include more frequent clinic appointments to monitor medication adherence and viral load, as well as strategies used in effective adherence EBIs, such as individual adherence counseling in clinical, community, or home settings, and adherence reminders. Furthermore, recent research highlights great interest in long-acting alternatives to daily antiretroviral regimens among AYA living with HIV [57]. Multiple long-acting injectable agents are in the final stages of development and warrant prioritization from the Food and Drug Administration for approval in treating AYA [56].
III. (a) AYA who are at risk and have been tested for HIV but are not on PrEP. Many important barriers to PrEP uptake and continuation among AYA warrant consideration. Inadequate PrEP awareness among providers remains an issue, particularly among generalists [45,58]. It is important that both primary care and HIV specialty care providers offer PrEP to AYA who are at risk and educate AYA on the availability of PrEP in clinical settings. In addition, perceived stigma represents a major barrier to PrEP uptake among AYA [59]. Service delivery in a stigma-free environment that is welcoming to AYA from racial, ethnic, and sexual minorities is important. Furthermore, many AYA who are at risk are in need of behavioral health services in the form of integrated service delivery or referrals, including those to address mental health and substance use problems [59]. Finally, the development of multipurpose prevention technologies that simultaneously prevent unintended pregnancies, STIs, and HIV and represent alternatives to PrEP may increase the uptake of biomedical HIV prevention among AYA [60].
(b) AYA living with HIV who are receiving HIV care but are not virally suppressed. In addition to standard HIV care and adherence support, care plans for AYA who are virally unsuppressed require a focus on services that address health and social barriers to initiating or continuing ART. Common barriers to treatment initiation or continuation of ART include stigma, trauma, mental health conditions, and substance use, as well as concern about medication toxicities and side effects [61,62]. Individualized strategies to address barriers to treatment initiation may include linkage to resources, such as support groups, community-based organizations, and other ancillary services. While some comprehensive models for the integration of behavioral health services into SRH and HIV specialty care have been developed, such as the Dean Street framework in the United Kingdom [50], AYA-specific best practices and models for service integration warrant further exploration.
IV. (a) AYA who are at risk and have not been tested for HIV. AYA who are at risk and are unaware of their HIV status represent a primary target population for intensive outreach efforts that promote HIV testing and may involve community partners. Venue-based testing and index-partner testing are established strategies to reach high-risk populations and have been implemented by the CDC [63,64]. More recently, network-based HIV testing approaches in high-risk sexual and drug use social networks have been employed successfully [65]. It is important to note that routine HIV testing for AYA in primary care has been included in clinical practice guidelines [66]. HIV tests should include risk assessments and linkage to appropriate HIV prevention, including PrEP. Future research should further evaluate novel and promising approaches to increase HIV testing rates, including for AYA, such as home-based self-testing [67].
(b) AYA who are virally unsuppressed and living with HIV but are not receiving HIV care. Intensive efforts to engage and re-engage AYA who are living with HIV and not currently receiving HIV services are warranted. Engagement and re-engagement services may include patient tracking via telephone, e-mail, mail, and home visits, and individual counseling outside the clinical setting, such as in the home or the community. Given inadequate engagement- and retention-in-care rates among AYA, alternative options to HIV care services delivered exclusively in the clinical setting should be explored. Community-based SRH service delivery, such as that provided in health care vans, has successfully engaged populations facing high access barriers to traditional health care institutions [68]. Similar approaches may support efforts to engage and re-engage AYA who are living with HIV.
Generally, the AYA who are least successfully engaged in comprehensive HIV prevention and treatment require the most intensive services. It is important to note, however, that the primary purpose of differentiated care is to meet the unique individual needs of AYA who are at risk and living with HIV. Hence, consideration of factors beyond engagement, retention, adherence, and viral suppression is always necessary to determine the appropriate what, when, who, and where of differentiated HIV care.
Conclusion
Despite significant progress in the fight against HIV/AIDS in the United States, HIV prevention and treatment disparities in key populations remain a national public health concern. While new HIV diagnoses are increasing among people below the age of 30, dominant prevention and treatment paradigms inadequately consider the unique HIV service needs of AYA who are at risk or living with HIV. In previous sections, we characterized AYA disparities across the HIV prevention and treatment continuum, existing resources for improving HIV service delivery to key populations in the United States, and a novel AYA-centered differentiated care framework adapted to the unique ecological and developmental factors that shape engagement, adherence, and retention in HIV services among AYA in the United States.
To address the persistent treatment and prevention disparities that threaten efforts to end the HIV/AIDS epidemic in the United States, innovative and AYA-specific care models are sorely needed. A novel differentiated care approach to HIV service delivery for AYA represents one such model. Shifting the paradigm for AYA to differentiated HIV care is a promising approach that warrants implementation and evaluation as part of reinforced national efforts to end the HIV epidemic in the United States by 2030.
Join the conversation!
![]() Tweet this! Not all HIV services fit every patient. @theNAMedicine’s new #NAMPerspectives discussion paper calls for differentiated service delivery, especially for at-risk youth: https://doi.org/10.31478/201903a
Tweet this! Not all HIV services fit every patient. @theNAMedicine’s new #NAMPerspectives discussion paper calls for differentiated service delivery, especially for at-risk youth: https://doi.org/10.31478/201903a
![]() Tweet this! It is time to turn national attention to those left behind in the fight against HIV/AIDS—adolescents and young adults who are at the greatest risk of, or are living with, HIV. Read more in the latest #NAMPerspectives from @theNAMedicine: https://doi.org/10.31478/201903a
Tweet this! It is time to turn national attention to those left behind in the fight against HIV/AIDS—adolescents and young adults who are at the greatest risk of, or are living with, HIV. Read more in the latest #NAMPerspectives from @theNAMedicine: https://doi.org/10.31478/201903a
![]() Tweet this! Despite significant progress made in the fight against HIV/AIDS in the US, the amount of young people diagnosed with HIV continues to increase. Learn what this #NAMPerspectives has to say about this national public health concern: https://doi.org/10.31478/201903a
Tweet this! Despite significant progress made in the fight against HIV/AIDS in the US, the amount of young people diagnosed with HIV continues to increase. Learn what this #NAMPerspectives has to say about this national public health concern: https://doi.org/10.31478/201903a
![]() Tweet this! To fully address the treatment and prevention disparities that affect young people, innovation and specific care models are needed that can be tailored to patients with different needs. Read in #NAMPerspectives about this approach for specialized HIV care: https://doi.org/10.31478/201903a
Tweet this! To fully address the treatment and prevention disparities that affect young people, innovation and specific care models are needed that can be tailored to patients with different needs. Read in #NAMPerspectives about this approach for specialized HIV care: https://doi.org/10.31478/201903a
Download the graphics below and share them on social media!
References
- Population Division. 2018. Annual estimates of the resident population for selected age groups by sex for the United States, states, counties and Puerto Rico commonwealth and municipios: April 1, 2010 to July 1, 2017. Suitland, MD: United States Census Bureau.
- Griffin, R., W. H. Frey, and R. Teixeira. 2017. States of change: Demographic change, representation gaps, and challenges to democracy, 1980-2060. Washington, DC: Center for American Progress, Brookings Institution, Bipartisan Policy Center. Available at: https://www.brookings.edu/wp-content/uploads/2017/02/states-of-change-report-2017.pdf (accessed January 31, 2019).
- Martin, J. A., B. E. Hamilton, and M. J. K. Osterman. 2018. Births in the United States, 2017; data brief, no. 318. Hyattsville, MD: National Center for Health Statistics. Available at: https://www.cdc.gov/nchs/data/databriefs/db318.pdf (accessed January 31, 2019).
- Centers for Disease Control and Prevention (CDC). 2018. 2017 sexually transmitted diseases surveillance. Available at: https://www.cdc.gov/std/stats17/default.htm (accessed December 20, 2018).
- CDC. 2018. HIV among youth. Available at: https://www.cdc.gov/hiv/group/age/youth/index.html (accessed January 31, 2019).
- CDC. 2017. Diagnoses of HIV infection in the United States and dependent areas, 2017. HIV Surveillance Report 29. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed January 31, 2019).
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. N.d. Selected national HIV prevention and care outcomes. PowerPoint. Available at: https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-prevention-and-care-outcomes.pdf (accessed January 31, 2019).
- Febo-Vazquez, I., C. E. Copen, and J. Daugherty. 2018. Main reasons for never testing for HIV among women and men aged 15–44 in the United States, 2011–2015. National Health Statistics Reports.Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr107.pdf (accessed January 31, 2019).
- CDC. 2019. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Report 24(1). Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed January 31, 2019).
- CDC. 2018. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data United States and 6 dependent areas, 2016. HIV Surveillance Report 23(4). Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed January 31, 2019).
- CDC. 2017. Effectiveness of prevention strategies to reduce risk of acquiring or transmitting HIV. Available at: https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html (accessed January 31, 2019).
- Grant, R. M., J. R. Lama, P. L. Anderson, V. McMahan, A. Y. Liu, L. Vargas, P. Goicochea, M. Casapia, J. V. Guanira-Carranza, M. E. Ramirez-Cardich, O. Montoya-Herrera, T. Fernandez, V. G. Beloso, S. P. Buchbinder, S. Chariyalertsak, M. Schechter, L. Bekker, K. H. Mayer, E. G. Kallas, K. R. Amico, K. Mulligan, L. R. Bushman, R. J. Hance, C. Ganoza, P. Defechereux, B. Postle, F. Wang, J. J. McConnell, J. Zheng, J. Lee, J. F. Rooney, H. S. Jaffe, A. I. Martinez, D. N. Burns, and D. V. Glidden. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New England Journal of Medicine 363:2587-2599. https://doi.org/1056/NEJMoa1011205
- Hosek, S. G., B. Rudy, R. Landovitz, B. Kapogiannis, G. Siberry, B. Rutledge, N. Liu, J. Brothers, K. Mulligan, G. Zimet, M. Lally, K. H. Mayer, P. Anderson, J. Kiser, J. F. Rooney, C. M. Wilson, and the Adolescent Trials Network for HIV/AIDS Interventions. 2017. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. Journal of Acquired Immune Deficiency Syndromes 74(1):21-29. https://doi.org/10.1097/QAI.0000000000001179
- Anderson, P. L., D. V. Glidden, A. Liu, S. Buchbinder, J. R. Lama, J. V. Guanira, V. McMahan, L. R. Bushman, M. Casapia, O. Montoya-Herrera, V. G. Veloso, K. H. Mayer, S. Chariyalertsak, M. Schechter, L. G. Bekker, E. G. Kallas, R. M. Grant, and the iPrEx Study Team. 2012. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science Translational Medicine 4(151):151ra125. https://doi.org/10.1126/scitranslmed.3004006
- Cardo, D. M., D. H. Culver, C. A. Ciesielski, P. U. Srivastava, R. Marcus, D. Abiteboul, J. Heptonstall, G. Ippolito, F. Lot, P. S. McKibben, and D. M. Bell. 1997. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. New England Journal of Medicine 337:1485-1490. https://doi.org/10.1056/NEJM199711203372101
- Mayer, K. H., M. J. Mimiaga, M. Gelman, and C. Grasso. 2012. Raltegravir, tenofovir DF, and emtricitabine for postexposure prophylaxis to prevent the sexual transmission of HIV: Safety, tolerability, and adherence. Journal of Acquired Immune Deficiency Syndromes 59:354-359. https://doi.org/10.1097/QAI.0b013e31824a03b8
- Irvine, C., K. J. Egan, Z. Shubber, K. K. A. Van Rompay, R. L. Beanland, and N. Ford. 2015. Efficacy of HIV postexposure prophylaxis: Systematic review and meta-analysis of nonhuman primate studies. Clinical Infectious Diseases 60(suppl 3):S165-S169. https://doi.org/10.1093/cid/civ069
- Hosek, S. G., R. Landovitz, B. Rudy, B. Kapogiannis, G. Siberry, B. Rutledge, N. Liu, J. Brothers, J. Rooney, C. M. Wilson, and the Adolescent Trials Network for HIV/AIDS Interventions. 2016. An HIV pre-exposure prophylaxis (PrEP) demonstration project and safety study for adolescent MSM ages 15-17 in the United States (ATN 113). Talk presented at the 21nd International AIDS Conference, Durban, South Africa. Available at: http://www.natap.org/2016/IAC/IAC_41.htm (accessed January 31, 2019).
- Storholm, E. D., J. E. Volk, J. L. Marcus, M. J. Silverberg, and D. D. Satre. 2017. Risk perception, sexual behaviors, and PrEP adherence among substance-using men who have sex with men: A qualitative study. Prevention Science 18(6):737-747. https://doi.org/10.1007/s11121-017-0799-8
- Traeger, M. W., S. E. Schroeder, E. J. Wright, M. E. Hellard, V. J. Cornelisse, J. S. Doyle, and M. A. Stoove. 2018. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: A systematic review and meta-analysis. Clinical Infectious Diseases 67(5):676-686. https://doi.org/10.1093/cid/ciy182
- Allen, E., D. Krakower, K. Hsu, and L. Wisk. 2018. SAHM — annual meeting: Abstracts of platform research presentations (1–34): estimated prevalence of adolescents and young adults with indications for HIV pre-exposure prophylaxis in the United States, 2011-2015. Journal of Adolescent Health 62(2)(suppl):S1-S2. https://doi.org/10.1016/j.jadohealth.2017.11.006
- AIDSVu. 2018. AIDSVu adds 2017 state-level data visualizing PrEP users across the U.S. Available at: https://aidsvu.org/aidsvu-adds-2017-state-level-data-visualizing-prep-users-across-the-u-s (accessed January 31, 2019).
- Azar, A. 2019. Ending the HIV epidemic: A plan for America. HIV.gov blog. Available at: https://www.hiv.gov/blog/ending-hiv-epidemic-plan-america (accessed February 11, 2019).
- U. S. Department of Health and Human Services (HHS). 2019. Ending the HIV epidemic: A plan for America. Available at: https://www.hhs.gov/sites/default/files/ending-the-hiv-epidemic-fact-sheet.pdf (accessed February 11, 2019).
- Fauci, A. S., R. R. Redfield, G. Sigounas, M. D. Weahkee, and B. P. Giroir. 2019. Ending the HIV epidemic: A plan for the United States. JAMA 32(9):844-845. https://doi.org/10.1001/jama.2019.1343
- HHS. 2019. Clinical guidelines. Available at: https://aidsinfo.nih.gov/guidelines (accessed January 31, 2019).
- CDC. 2018. Compendium of evidence-based interventions and best practices for HIV prevention. Available at: https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html (accessed January 31, 2019).
- Garofalo, R., L. M. Kuhns, A. Hotton, A. Johnson, A. Muldoon, and D. Rice. 2016. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS and Behavior 20(5):1049-1059. https://dx.doi.org/10.1007%2Fs10461-015-1192-x
- Panel on Antiretroviral Guidelines for Adults and Adolescents. 2018. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Washington, DC: Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed January 31, 2019).
- Adolescent Medicine Trials Network for HIV/AIDS Interventions. ATN. Available at: https://www.nichd.nih.gov/research/supported/atn (accessed January 31, 2019).
- Hosek, S. G., G. W. Harper, D. Lemos, J. Burke-Miller, S. Lee, L. Friedman, and J. Martinez. 2018. Project ACCEPT: Evaluation of a group-based intervention to improve engagement in care for youth newly diagnosed with HIV. AIDS and Behavior 22(8):2650-2661. https://doi.org/10.1007/s10461-018-2034-4
- Adolescent Medicine Trials Network for HIV/AIDS Interventions. N.d. Current studies. Available at: https://atnweb.org/atnweb/studies (accessed January 31, 2019).
- Health Resources and Services Administration HIV/AIDS Bureau. 2018. Building futures: Supporting youth living with HIV technical assistance toolkit. PowerPoint. Available at: https://targethiv.org/sites/default/files/file-upload/resources/HRSA_HAB_Building_Futures_Technical_Assistance_Toolkit_0.pdf (accessed January 31, 2019).
- International AIDS Society. N.d. Differentiated service delivery. Available at: http://www.differentiatedcare.org (accessed January 31, 2019).
- Armstrong, A., J. M. Nagata, M. Vicari, C. Irvine, L. Cluver, A. H. Sohn, J. Ferguson, G. Caswell, L. W. Njenga, C. Oliveras, D. Ross, T. Puthanakit, R. Baggaley, and M. Penazzato. 2018. A global research agenda for adolescents living with HIV. Journal of Acquired Immune Deficiency Syndromes 78(1)(suppl):S16-S21. https://dx.doi.org/10.1097%2FQAI.0000000000001744
- Duncombe, C., S. Rosenblum, N. Hellmann, C. Holmes, L. Wilkinson, M. Biot, H. Bygrave, D. Hoos, and G. Garnett. 2015. Reframing HIV care: Putting people at the centre of antiretroviral delivery. Tropical Medicine & International Health 20(4):430-447. https://dx.doi.org/10.1111%2Ftmi.12460
- International AIDS Society. VMMC outreach and partner notification with self-test kits. Available at: http://www.differentiatedcare.org/Models/Self-Testing/VMMC-Outreach-PartnerNotification (accessed January 31, 2019).
- International AIDS Society. Youth clubs. http://www.differentiatedcare.org/Models/YouthClubs (accessed January 31, 2019).
- Reif, L. K., M. L. McNair, M. R. Lamb, and B. Elul. 2018. Youth-friendly services and differentiated models of care are needed to improve outcomes for young people living with HIV. Current Opinion in HIV and AIDS 13(3):249-256. https://doi.org/10.1097/COH.0000000000000454
- Miller, R. L., T. Strzyzykowski, K. S. Lee, D. Chiaramonte, I. Acevedo-Polakovich, H. Spring, O. Santiago-Rivera, C. B. Boyer, and J. M. Ellen. 2018. Structural effects on HIV risk among youth: A multi-level analysis. AIDS and Behavior 22(11):3451-3467. https://doi.org/10.1007/s10461-018-2031-7
- Horn, T., J. Sherwood, R. H. Remien, D. Nash, J. D. Auerbach, and the Treatment Action Group and Foundation for Aids Research HIV Prevention Continuum Working Group. 2016. Towards an integrated primary and secondary HIV prevention continuum for the United States: A cyclical process model. Journal of the International AIDS Society 19(1):21263. https://doi.org/10.7448/IAS.19.1.21263
- Reisner, S. L., L. Jadwin-Cakmak, J. M. W. Hughto, M. Martinez, L. Salomon, and G. W. Harper. 2017. Characterizing the HIV prevention and care continua in a sample of transgender youth in the US. AIDS and Behavior 21(12):3312-3327. https://doi.org/10.1007/s10461-017-1938-8
- McNulty, M. C., and J. A. Schneider. 2018. Care continuum entry interventions: Seek and test strategies to engage persons most impacted by HIV within the United States. AIDS 32(4):407-417. https://doi.org/10.1097/QAD.0000000000001733
- CDC and US Public Health Service. 2018. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: A clinical practice guideline. Washington, DC: Department of Health and Human Services; Atlanta, GA: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf (accessed February 11, 2019).
- Mayer, K. H. 2019. HIV now: Overcoming barriers to PrEP uptake in the United States. Webinar. Available at: https://events.clinicaloptions.com/hiv/event-list/event/?id=aab86da1-421f-e911-80e5-005056943d50 (accessed January 30, 2019).
- Touger, R., and B. R. Wood. 2019. A review of telehealth innovations for HIV pre-exposure prophylaxis (PrEP). Current HIV/AIDS Reports 1-7. https://doi.org/10.1007/s11904-019-00430-z
- Siegler, A. J., K. H. Mayer, A. Y. Liu, R. R. Patel, L. M. Ahlschlager, C. S. Kraft, R. Fish, S. E. Wiatrek, and P. S. Sullivan. 2019. Developing and assessing the feasibility of a home-based preexposure prophylaxis monitoring and support program. Clinical Infectious Diseases 68(3):501-504. https://doi.org/10.1093/cid/ciy529
- Futterman, D., B. Chabon, and N. D. Hoffman. 2000. HIV and AIDS in adolescents. Pediatric Clinics of North America 47(1):171-188. https://doi.org/10.1016/S0031-3955(05)70200-5
- Spiegel, H. M. L., and D. C. Futterman. 2009. Adolescents and HIV: Prevention and clinical care. Current HIV/AIDS Reports 6(2):100-107. https://doi.org/10.1007/s11904-009-0015-y
- Stuart, D., and L. Chislett. 2016. The Dean Street Wellbeing programme: Culturally tailored community engagement programmes to combat a challenging epidemic. HIV Nursing 16:7-10. Available at: https://www.academia.edu/24252581/The_Dean_Street_Wellbeing_programme_culturally_tailored_community_engagement_programmes_to_combat_a_challenging_epidemic (accessed September 2, 2020).
- Hosek, S. G., G. W. Harper, and W. L. Robinson. 2002. Identity development in adolescents living with HIV. Journal of Adolescence 25(4):355-364. https://doi.org/10.1006/jado.2002.0480
- Bruce, D., G. W. Harper, and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. 2012. Future life goals of HIV-positive gay and bisexual male emerging adults. Journal of Adolescent Research 27(4):449-470. https://doi.org/10.1177/0743558411417870
- National Institutes of Health. 2015. Functional wellness in HIV: Maximizing the treatment cascade (R21); funding opportunity announcement, no PA-15-134. Available at: https://grants.nih.gov/grants/guide/pa-files/PA-15-134.html (accessed February 11, 2019).
- Liu, A. Y., E. Vittinghoff, P. von Felten, K. Rivet Amico, P. L. Anderson, R. Lester, E. Andrew, I. Estes, P. Serrano, J. Brothers, S. Buchbinder, S. Hosek, and J. D. Fuchs. 2018. Randomized controlled trial of a mobile health intervention to promote retention and adherence to preexposure prophylaxis among young people at risk for human immunodeficiency virus: The EPIC Study. Clinical Infectious Diseases. https://doi.org/10.1093/cid/ciy810
- Molina J. M., J. Ghosn, L. Beniguel, D. Rojas-Castro, M. Algarte-Genin, G. Pialoux, C. Delaugerre, Y. Yazdanpanah, C. Katlama, C. Segouin, S. Morel, C. Pintado, B. Loze, S. Le Mestre, S. Gibowski, V. Dore, L. Assoumou, B. Spire, D. Costagliola, and the Prevenir ANRS Study Group. 2018. Incidence of HIV-infection in the ANRS Prevenir Study in the Paris region with daily or on demand PrEP with TDF/FTC. Talk presented at 22nd International AIDS Conference, Amsterdam, Netherlands. Available at: http://www.natap.org/2018/IAC/IAC_32.htm (accessed February 11, 2019).
- AmfAR. 2018. Long-acting HIV treatment and prevention are coming: Preparing for potential game changers. Available at: https://www.amfar.org/uploadedFiles/_amfarorg/Articles/On_The_Hill/2018/chart.pdf (accessed February 11, 2019).
- Biello, K. B., M. J. Mimiaga, C. M. Santostefano, D. S. Novak, and K. H. Mayer. MSM at highest risk for HIV acquisition express greatest interest and preference for injectable antiretroviral PrEP compared to daily, oral medication. AIDS and Behavior 2(4):1158-1164. https://doi.org/10.1007/s10461-017-1972-6
- Mullins, T. L. K., and C. E. Lehmann. 2018. Oral pre-exposure prophylaxis (PrEP) for HIV prevention in adolescents and young adults. Current Pediatrics Reports 6(2):114-122. https://doi.org/10.1007/s40124-018-0163-x
- Doll, M., J. D. Fortenberry, D. Roseland, K. McAuliff, C. M. Wilson, and C. B. Boyer. 2018. Linking HIV-negative youth to prevention services in 12 US cities: Barriers and facilitators to implementing the HIV prevention continuum. Journal of Adolescent Health 62(4):424-433. https://doi.org/10.1016/j.jadohealth.2017.09.009
- Young Holt, B., L. Dellplain, M. D. Creinin, K. J. Peine, J. Romano, and A. Hemmerling. 2018. A strategic action framework for multipurpose prevention technologies combining contraceptive hormones and antiretroviral drugs to prevent pregnancy and HIV. European Journal of Contraception & Reproductive Health Care 23(5):328-334. https://doi.org/10.1080/13625187.2018.1508650
- Dombrowski, J. C., J. M. Simoni, D. A. Katz, and M. R. Golden. 2015. Barriers to HIV care and treatment among participants in a public health HIV care relinkage program. AIDS Patient Care and STDs 29(5):279-287. https://doi.org/10.1089/apc.2014.0346
- Gwadz, M., E. Applegate, C. Cleland, N. R. Leonard, H. Wolfe, N. Salomon, M. Belkin, M. Riedel, A. Banfield, L. Sanfilippo, A. Wagner, D. Mildvan, and the Heart to Heart Collaborative Research Team. 2014. HIV-infected individuals who delay, decline, or discontinue antiretroviral therapy: Comparing clinic-and peer-recruited cohorts. Frontiers in Public Health 2:81. https://doi.org/10.3389/fpubh.2014.00081
- CDC. 2018. HIV testing in nonclinical settings. Available at: https://www.cdc.gov/hiv/testing/nonclinical/index.html (accessed February 11, 2019).
- Stein R., W. Song, M. Marano, H. Patel, S. Rao, and E. Morris. 2017. HIV testing, linkage to HIV medical care, and interviews for partner services among youths — 61 health department jurisdictions, United States, Puerto Rico, and the U.S. Virgin Islands, 2015. MMWR Morbidity and Mortality Weekly Report 66(24):629-635. http://dx.doi.org/10.15585/mmwr.mm6647e1
- Nikolopoulos, G. K., E. Pavlitina, S. Q. Muth, J. Schneider, M. Psichogiou, L. D. Williams, D. Paraskevis, V. Sypsa, G. Magiorkinis, P. Smyrnov, A. Korobchuk, T. I. Vasylyeva, B. Skathun, M. Malliori, E. Kafetzopoulos, A. Hatzakis, and S. R. Friedman. 2016. A network intervention that locates and intervenes with recently HIV-infected persons: The Transmission Reduction Intervention Project (TRIP). Scientific Reports 6:38100. https://doi.org/10.1038/srep38100
- Branson, B. M., H. H. Handsfields, M. A. Lampe, R. S. Janssen, A. W. Taylor, S. B. Lyss, and J. E. Clark. 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Morbidity and Mortality Weekly Report: Recommendations and Reports 55(14):1-17. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/Rr5514a1.htm
- Clark, H. A., E. Oraka, E. A. DiNenno, L. G. Wesolowski, P. R. Chavez, M. A. Pitasi, and K. P. Delaney. 2018. Men who have sex with men (MSM) who have not previously tested for HIV: Results from the MSM Testing Initiative, United States (2012–2015). AIDS and Behavior 23(2):359-365. https://doi.org/10.1007/s10461-018-2266-3
- Liebman, J., M. Pat Lamberti, and F. Altice. 2008. Effectiveness of a mobile medical van in providing screening services for STDs and HIV. Public Health Nursing 19(5):345-353. https://doi.org/10.1046/j.1525-1446.2002.19504.x
Chronic Disease, Diversity and Inclusion, Health Disparities, Health Equity, Public Health