Redesigning Provider Payments to Reduce Long-Term Costs by Promoting Healthy Development
ABSTRACT | Cognitive, affective, and behavioral health (CAB) conditions are among the costliest and fastest growing in the United States. An array of interventions is demonstrated to be effective in preventing or mitigating these conditions and offers the possibility of lower costs and improved lifelong health. These effective interventions have not been widely integrated into health care, and current health care reform efforts have spurred limited additional uptake. Redesigning incentives to maximize life course CAB health is critical to reducing health costs and improving population health. Future health care reform efforts will need to redesign incentives by developing quality measures of CAB developmental outcomes for accountability, creating payment methodologies based on the expected value of changes in these outcomes, and ensuring sufficient reimbursement. These three changes would allow for timely incentives for effectively promoting life course CAB health and potentially reducing future health system spending. Health care reforms will also need to engage other sectors that contribute to and help optimize CAB health, including child care and education.
Introduction
Policy makers, administrators, and clinicians face increasing demands to achieve the triple aim by reducing health care costs while improving quality and population health outcomes. A growing body of research indicates that achieving sustainable gains in health and lowering costs will require more effective “upstream” preventive and population-based interventions, which interrupt causal pathways to chronic long-term health conditions and optimize healthy development across the lifespan [1]. To produce greater long-term savings and better health, U.S. health care systems will need to accelerate the implementation of effective prevention and population health improvement strategies.
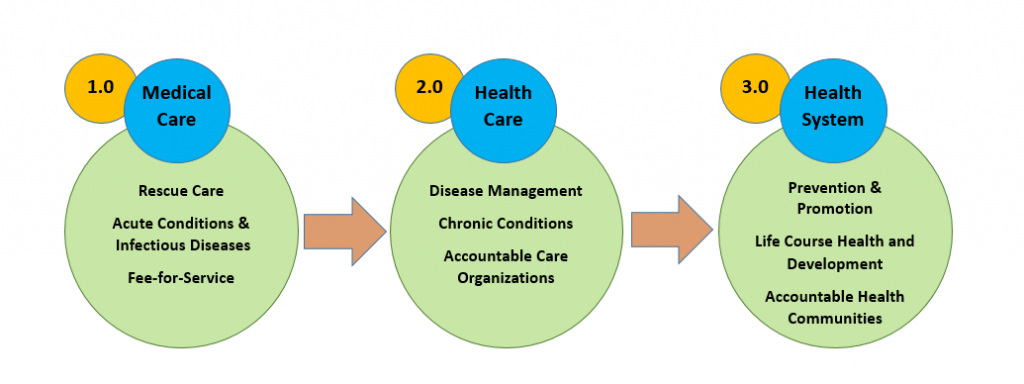
One useful way to characterize and inform this transformative shift is through the 3.0 Transformation Framework (TF) [2]. The 3.0 TF describes the drivers contributing to the intermittent evolution of U.S. health care delivery systems over the past century. The progression began with a 1.0 medical care system that focused on rescue care for those with acute conditions and infectious diseases, and evolved to the current 2.0 health care system, largely focused on creating networks initially like managed care and moving to accountable health care organizations that manage ever-increasing rates of chronic disease (see Figure 1). This shift from the first-era system to the second-era system was driven by epidemiologic changes in mortality and morbidity (from communicable to noncommunicable diseases); scientific advances that helped to replace simple, linear biomedical models with a more complex and nuanced biopsychosocial framework for understanding health; a bevy of new technologies, strategies, and organizational advances; and growing financial constraints and demands for fiscal accountability.
Figure 1 | The Transformation Framework
Today, the evolutionary pressures fueling a shift to a third-era 3.0 health system are already underway. Life course health science is transforming how we understand the epigenetic and developmental origins of many lifelong conditions, and we are witnessing a dramatic shift in the salience of conditions such as obesity, behavioral and mental health conditions, and substance use, which have complex, multilevel ecological, social, behavioral, and developmental determinants [3]. With a focus on optimizing lifelong health and achieving equity from the start, 3.0 transformative innovations are already prioritizing strategies that advance upstream prevention and health promotion at the individual, population, and community levels [4]. We see these first stages in cross-sector health improvement strategies that address truly upstream socioeconomic drivers of health and health care costs, such as built environment and poverty [5], as well as in health care models such as the Accountable Health Communities Model [6], the Diabetes Prevention Program [7], and the Vermont All-Payer Accountable Care Organization Model [8], which lay the foundation for integrating life course and health equity perspectives into future payment and delivery models. Note that while the current movement represents a new, system-wide effort to address population health in payment systems, innovative providers have been implementing effective prevention and promotion interventions for decades. Unfortunately, the 1.0 and 2.0 health payment systems did not foster these efforts, and many of the most innovative interventions that one might expect in a 3.0 system have been largely grant-funded, making system-wide progress impractical until incentive structures are redesigned and larger payment reforms are underway.
While initial results of payment reforms have been promising, most of these initiatives have failed to meaningfully address some of the nation’s largest cost drivers: cognitive, affective, and behavioral (CAB) health problems, such as behavioral and mental health conditions, self-injury and suicide, substance use disorders and overdoses, obesity and chronic diseases, risky driving and accidental injuries, unintended pregnancies and sexually transmitted infections, autism spectrum disorders, and premature births [9]. CAB health conditions have become the costliest in the United States [10]. These conditions are also leading causes of disability in the United States and are frequently interrelated with other chronic physical health conditions, including heart disease, hypertension, and diabetes [11]. Many CAB symptoms and disorders emerge during childhood, often from the impact of adverse experiences, social inequities, and environmental stressors. These early experiences can impact the trajectory of brain development and increase rates of CAB health conditions and interrelated chronic diseases later in life [12]. Interventions to promote CAB health, especially in childhood and adolescence, offer the possibility of substantial savings across the course of an individual’s life.
As the burden of chronic conditions related to CAB development increases, we find mounting evidence that it is possible to prevent or mitigate these conditions in individuals, in general populations, and in subpopulations that have experienced historical health disparities. While many preventive interventions are condition-specific, some address common risk factors and can prevent multiple CAB-related health conditions by promoting healthy CAB development [13,14]. The specifics of each intervention differ, but they tend to function by helping families, schools, or communities to structure interactions and the environment in ways that meet children’s specific CAB developmental needs, and/or by focusing on the child or children directly to help build core CAB developmental competencies that allow the child or children to successfully manage developmental transitions and environmental challenges in family, school, and community life. A number of these interventions are considered evidence-based, have been successfully implemented at a number of sites, and have appropriate scale-up plans that make them ready for broad implementation [15]. Of the interventions appropriate for scale-up, some have demonstrated health care cost savings over the long-term [16], and others can be adapted using technology or other strategies to reduce the costs and increase the return-on-investment ratio [17,18,19]. Wide-scale implementation of interventions that promote healthy CAB development—whether directed toward individuals, families, classrooms, or broader communities—has the potential to substantially decrease the cost of health care and improve the health of the population over the long term. As one critical site for near-universal access in a child’s first years of life, the health care system must be configured to provide interventions that promote healthy CAB development.
Unfortunately, the current health care payment structure not only fails to provide appropriate upstream incentives but often does exactly the opposite by creating disincentives for CAB health promotion and risk prevention. Consequently, few of the effective interventions for promoting CAB health have made it beyond research and into clinical practice [20]. For example, in the Quality Payment Program of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), value-based payments may accrue when a provider screens for depression and creates a follow-up plan, or when effective treatment interventions achieve progress toward remission. Incentive payments do not accrue, however, when a provider intervenes to prevent depression or its recurrence [21,22,23]. This dysfunctional misalignment of incentives is systemic throughout health care sectors and runs counter to the strategies that are necessary to achieve transformation to a 3.0 health system. With appropriate incentives in place for CAB health promotion, health systems could offer effective prevention in CAB health, with the potential for reductions in suffering and some of the related health care expenses.
This article (1) examines a possible trajectory of health care reform and the potential of leading health care payment and delivery systems to more strategically leverage the promotion of healthy CAB development; (2) outlines three areas that will need to be addressed to create incentives for promoting CAB health—quality measurement, appropriate incentive payments, and sufficient reimbursement; and (3) reviews other work that will be required to maximize CAB health and progress toward a 3.0 TF.
The Trajectory of Health Care Payment and Delivery Reform
Although impending policy changes will alter details, the tectonic shift from volume-based payment toward value-based and population-based payment models is expected to continue for the foreseeable future, opening up opportunities to redesign health care payment incentives to promote CAB health [24].
“Value-based payment” generically refers to arrangements in which payers reimburse health care providers or provider groups based on their performance on different indicators, often a mix of quality and cost measures. Population-based payment is one type of value-based payment arrangement in which payers reimburse health care providers based on the estimated cost of effectively managing the health of a population of individuals, with incentives for quality, rather than reimbursing for each service provided to the population. These payment arrangements could offer a health care system the financial incentives and flexibility to maximize health status across many—if not most—populations.
Efforts toward these types of payment reforms have been, for the most part, bipartisan and have spanned public and private sectors. MACRA, the pivotal legislation on value-based payment and alternative payment models (APMs), received bipartisan support in 2015. While some private sector initiatives toward innovative payment arrangements have occurred in concert with public sector support, other private sector initiatives have been entirely independent. For example, Blue Cross Blue Shield of Michigan began a value-based payment initiative in 2004, years before most federal value-based payment programs, and has since expanded across a number of provider types and settings [25]. Health care delivery system and payment reforms will continue to evolve over the coming years, guided by value-based and population-based payments goals, given the bipartisan support and substantial private sector buy-in.
In some instances, the proliferation of payment innovations has already enabled the implementation of some interventions that promote CAB health [26]. Unfortunately, despite these scattered successes, health care reform efforts as a whole are not on track to meaningfully incentivize prevention and promotion efforts in CAB health at the provider level. Provider-level financing for CAB development is challenged by at least three issues: (1) better quality measurement to link to incentives, (2) appropriate incentive payments, and (3) sufficient reimbursement. Better quality measures that are aligned with CAB payment and outcome strategies will be needed to document the effectiveness of CAB interventions in diverse populations and settings. Health systems will need to offer appropriate incentive payments for achieving CAB quality outcomes. Finally, providers will need sufficient reimbursement to perform the interventions that affect the agreed upon quality measures.
Quality Measurement
Both population-level and provider-level measures will be necessary to advance incentives for CAB health promotion. Population-level outcome measures allow stakeholders to assess the well-being of a large group of people using representative indicators, whereas provider-level outcome measures track the short-term health of individuals receiving care using indicators relevant to the provider. The provider-level measures drive changes in a provider’s practice (e.g., whether they deliver interventions to promote CAB health), while population-level measures drive changes at the system level (e.g., whether communities build systems of support for early childhood, which is especially important for addressing health equity across a community). While some strong population-level measures for CAB development have been created and used for community-wide intervention efforts [27,28], there has been limited progress toward developing and validating appropriate provider-level outcome measures for CAB health [29]. (Note that, although “provider” often refers to a clinician in health care payment reform, the provider being incentivized need not be a clinician. “Provider,” as used here, refers to any individual compensated by a health care payer to deliver any part of an intervention to improve health, and so the term could include community health workers, peer support specialists, or other types of practitioners.)
In determining what measures might be most appropriate, the literature makes a useful distinction among three types of health: health conditions, functioning, and health potential. The latter refers to “the development of health assets that indicate positive aspects—competence, capacity, and developmental potential [30].” In CAB health, health potential refers to the development of social, emotional, and behavioral competencies, which may be defined as
[A] family of constructs related to the capacity or motivation for, process of, or outcomes of effective adaptation in the environment, often inferred from a track record of effectiveness in age-salient developmental tasks and always embedded in developmental, cultural and historical context [31].
While all development occurs in relation to others, child development in particular should also be contextualized within the caregiver(s)-child interactions in the family and educational systems that build these developmental competencies. Thus, developmental competencies in the child, the dynamics with caregivers as they relate to the child, and the functioning of health supportive systems such as communities and schools together constitute health potential for the child’s CAB health. Health potential related to CAB development could be measured in health care at both the population and provider levels to predict some types of changes in health conditions and functioning that will manifest years later, far beyond the time frame in which incentive payments are typically offered.
Beyond this conceptual framework, there is little consensus around which measurable constructs capture developmental competencies or how these competencies should be measured [12]. To ensure that appropriate measures are used in value-based or population-based health care systems, two strategies must be employed. First, health care systems must experiment with existing measures in value-based and population-based payment frameworks to determine if they effectively incentivize CAB prevention and promotion. Second, over the next decade, clinically appropriate measures must be developed for public use to evaluate the impact of CAB prevention and promotion services, and determine how best to effectively integrate CAB prevention and promotion into different clinical and community contexts.
Experimentation with existing measures in emerging value-based and population-based payment frameworks can drive stronger incentives for prevention and promotion in the near future. Existing CAB developmental measurement tools should be tested for use as quantitative scales. In such testing, healthy development and the effectiveness of interventions would be evaluated by changes in the score on the measurement tool. Some measurement tools have been tested as quantitative scales in this way [32], but none have been used in the high-stakes context of value-based and population-based payments, where payments are tied to risk-adjusted improvements or maintenance of high scores on the measurement tool. In addition, in keeping with the emerging movement toward a two-generation approach to health, appropriate measures for parents will also be needed [33]. The measures used for parents should be expanded beyond current efforts to screen for maternal depression, as so much of healthy CAB development involves engaging parents in addressing an array of CAB needs. Providers could look at both parental risk factors (such as parental stress or maladaptive coping behaviors [e.g., parental substance use]) [34] and parental competencies and functioning associated with healthy CAB development (such as good parent-child communication) [35]. Those measures that prove helpful in directing and evaluating interventions in health and health care could then be used in value-based payment.
While existing measures offer the possibility of some progress, the measure development pipeline should prioritize measures of healthy CAB development to ensure that health systems use the most effective measures for incentivizing prevention and promotion. The Centers for Medicare and Medicaid Services and the National Quality Forum should propose child and parent CAB measures for each stage of development as priority gaps to be addressed and as an area of focus for the Pediatric Quality Measures Program of the Children’s Health Insurance Program Reauthorization Act of 2009. There are many clinical research measurement initiatives that could lay the foundation for building a clinical quality measure set, such as the National Institutes of Health (NIH) Toolbox [36], the NIH Patient Reported Outcome Measurement Information System (PROMIS) measure set and the multivariate computerized adaptive tests [37], the NIH’s PhenX initiative [38], and the National Institute of Mental Health’s Research Domain Criteria (RDoC) [39]. Fewer initiatives have focused on individual- and community-level risk and protective factors for use in health care (with a notable exception in Vital Signs, from the National Academy of Medicine, which proposes population markers of developmental progress, such as kindergarten readiness, teen pregnancy, and high school graduation [40,41]) or relational health, such as in a child-caregiver dyad in the first few years of life through adolescence). The ultimate measures of CAB health potential may include a combination of the psychometrically valid person-reported outcome measure sets, such as the PROMIS set (which has constructs like “Depression” and “Applied Cognitive Abilities”), and the neuroscience-derived measure sets, such as the RDoC set (which has domains like “Frustrative Non-Reward” and “Declarative Memory”), along with individual- and community-level risk and protective factors and relational measures—the latter of which may be as necessary to understanding developmental trajectory as any individual-level measure. All of this measure development will need to consider the overall measurement burden and the opportunities afforded by technologically efficient data collection systems (such as computerized adaptive testing [42]) and/or remote reporting through active or passive systems [43].
Clinical and community measurement development initiatives could fuel research on efficient measures that can be tracked over the near term but effectively predict the impacts of prevention and promotion over the longer term—and eventually fuel practice.
Appropriate Incentive Payments to Achieve Better Longitudinal Integration
With quality measures in place to track CAB development, value-based payments can be provided for achieving improvements in individual-level measures over time, in much the same way that MACRA and many APMs currently offer provider incentives. However, MACRA and existing APM incentive structures represent a partial transition from volume to value—they pay for value in the context of the specific health need but not for the value to overall life course health. To promote the most efficient allocation of resources for population health, incentives should be tied to the amount of value the intervention confers across the individual’s life course. Note that benefits also accrue to sectors outside of health care, such as juvenile justice, so that value under this framework would not be fully captured by a financing model that engaged the health sector alone. This “wrong pocket problem,” where interventions delivered in one sector lead to savings in another, is addressed later in this paper.
MACRA and APMs do include incentives for the efficient allocation of resources, usually in the form of total cost of care (TCOC) over the time frame under evaluation. TCOC is designed to ensure that health care systems are achieving health outcomes at the lowest costs. TCOC could be improved as a measure of efficient allocation of resources if it included forward-looking elements that incorporate how the health care system’s present allocations of resources affect future health outcomes and costs of care. Incentives that consider the net present value of care would favor the implementation of interventions that are most cost-effective to Americans, such as prevention, children’s health promotion services, and other population health investments. This would shift the current distribution of spending away from adult chronic disease management and rescue care, and toward those services and supports in childhood that are most likely to mitigate later needs. Note that, as the wrong pocket problem discussed later in this paper indicates, TCOC should be expanded as appropriate to include the costs from other health-producing systems.
Longitudinal studies of mediating factors associated with later health outcomes can provide a compelling starting point for redesigning TCOC. For example, social-emotional kindergarten readiness significantly predicts reduced psychiatric medication usage by age 25 [44]. This research allows the expected value of improvements in kindergarten readiness to be determined from anticipated savings in health service utilization, and used to begin to calculate net present value-based payment amounts for a life course TCOC incentive [45]. Over time, as all-payer claims databases are increasingly implemented and data from other sectors can be integrated, actuarial science can provide more robust estimates of life course TCOC using large sets of life course socioeconomic and health data to more accurately predict changes in future costs of care. Although these robust predictions may take some time to evolve, payment pilots can begin immediately in places where local payer and provider capacities align.
An incentive system that rewards positive changes in the trajectory of CAB development and reduction of life course TCOC would also inherently adjust for risk, promoting equity. By paying for dimensional improvements in CAB health potential and expected reductions in health service utilization over time, providers could receive the largest incentive payments for the highest risk children and much lower incentive payments for low-risk children. Throughout implementation, incentives should be further structured to address the fundamental goal of achieving health equity, with services organized to reduce disparities in health, health services, and the social determinants, and to overcome historical inequities that underlie many of these disparities.
Policy changes will be necessary to implement a life course TCOC incentive framework. Health plans have several financial disincentives to pay for the preventive care of individuals at risk. The most notable disincentive is that the current TCOC configuration mirrors the incentives that health plans face: individuals are free to and often do leave a given health plan after a few years. If the savings accrue outside of that time frame, the benefits accrue to another health plan, removing any financial incentive to invest in prevention. This presents a classic collective action problem: health plans benefit if they all pay for preventive interventions, but a health plan loses if it is the only one that pays. Legislation that mandates preventive services, such as those identified by the US Preventive Services Task Force; contract terms in all-payer arrangements; or some payer-provider collaborations could require the use of a common life course TCOC incentive structure to address this issue and establish value-based payment rates for performance on quality measures of promoting healthy CAB development [46].
Sufficient Reimbursement
Health systems will need to do more than apply new incentives to usual care to effectively promote CAB health and reduce costs.
Fee-for-service (including managed care), as currently structured, is generally not conducive to the provision of CAB prevention and promotion interventions. In fee-for-service settings, providers currently have limited opportunity to take advantage of value-based incentive payments, as there are limited billing codes for such health promotive services, and providers face competing professional accreditation and site certification requirements. Even in many current population-based payment frameworks, the case rate is often based on the current fee-for-service framework and so will be insufficient for providers to promote CAB health. Appropriate base reimbursement will be required for providers to implement effective interventions, receive the associated incentive payments for promoting CAB health, and produce the associated downstream savings.
To recalibrate the base reimbursement rate and test new incentive models, health systems should begin by implementing family-focused preventive interventions in the context of an integrated (behavioral, developmental, and physical health) and interdisciplinary care setting [47]. Family-focused preventive interventions are evidence-based interventions that engage parents, sometimes in groups, to improve their children’s healthy CAB development. Some family-focused interventions are offered universally to promote health, whereas others are offered selectively to address specific risk factors. There is a large body of research demonstrating these interventions’ long-term effectiveness and impact on health equity, as well as the cost-effectiveness of a number of different family-focused interventions in nonmedical settings. There is also growing momentum around their widespread implementation, including in primary health care [20]. Most recently, Washington State created billing codes for a short course of one of these interventions, the Triple P Positive Parenting Program, and allowed certified providers in a number of pilot sites to bill for it [48]. If reimbursable codes for family-focused interventions were created for use—both universally and selectively, as appropriate—in an integrated behavioral health and primary care practice, providers would have a strong starting point to promote healthy CAB development and receive corresponding incentive payments for decreasing long-term costs.
By expanding these types of models, and pairing those with the incentive systems outlined above, health systems can begin to experiment with the most effective financing systems to promote CAB health and achieve larger reductions in overall health care costs. To ensure that the additional upfront expenditures in implementing these models ultimately result in downstream savings, payers and providers can work together to ensure that enhanced reimbursements do not exceed the expected value of the services provided, as calculated by the life course TCOC. When providers scale CAB promotion models, they will need to experiment with different provider types offering services, simplifying interventions, and employing technology and telehealth as appropriate to achieve desired outcomes within the financial constraints of health care systems.
Issues in Cross-Sector Integration
To transition to a 3.0 health optimizing system, incentive realignment must look beyond health care and take an ecological perspective across all systems that produce health. This “360 degree view” would align across institutions, including child care, education, juvenile justice, faith-based groups, employers, social services, community organizations, home visitation programs, and others, with each of these stakeholders sharing common outcomes and planning processes [1]. Cross-sector integration and alignment is especially important for CAB health, as other sectors beyond health care spend an appreciable amount of time with children and families and have the opportunity to reinforce and amplify interventions to promote healthy CAB development [49, 50]. CAB health is also important for other sectors, as effective CAB promotion interventions demonstrate effects on academic achievement, juvenile justice involvement, and social service use. We anticipate that the ideal incentives for such cross-sector integration will need to track improvements in population-level CAB and major milestones (e.g., school readiness, third grade reading competency).
Financing and incentive structures will need to reinforce this cross-sector alignment. Many of the benefits of healthy CAB development accrue to sectors outside of health care—such as reduced spending on criminal justice, special education, and child welfare, and increased tax revenue from increased worker productivity. This reduces a health plan’s incentive to invest in prevention and promotion because it sees less of the benefit, an issue commonly referred to disparagingly as the wrong pocket problem. Policies should begin to experiment with ways to allow health care systems to share in cross-sector savings, providing stronger incentives for the health care sector to invest in healthy CAB development. Similarly, community partners should receive incentives for their contributions toward healthy CAB development. Early care and education, schools, and community programs all have the capacity to positively impact CAB health and should receive financial incentives to do so, including appropriate reimbursement for the value they add.
As health care systems adopt 3.0 integration strategies that lead to co-development and co-design of services with other sectors and systems, federal, state, and local policies should implement braided and blended funding models with shared accountability systems to provide fair rewards for cooperation and co-investment. Appropriately balanced incentives should ensure that the health care system plays a support role to other sectors where appropriate and does not subsume them, building an ecosystem where different community stakeholders are empowered as co-producers of CAB health. Ideally, the additional resources made available from cross-sector savings should be fairly distributed across the entities that contributed to their production.
Conclusion
Under mounting pressure to reduce the costs of health care to Americans, leaders in health and health care must accelerate current progress in the fundamental transformation of how services are organized, integrated, and compensated. Reductions in the burden of the costliest health conditions are possible by using 3.0 design strategies for transforming health care systems so they can more effectively promote population and individual CAB health. To realize the potential impact of optimizing CAB health as part of emerging health care reform strategies, quality measures of CAB health in value-based payment must be tested. Simultaneously, strategies to redesign total cost of care to include future health care costs must be advanced to justify sufficient reimbursement and incentive payments for interventions that promote CAB health. Redesigning incentives in health care and across sectors to maximize healthy CAB development across the life course and reduce health inequities is a crucial step in reducing the costs of health care and promoting the health of the American population [51].
Join the conversation!
![]() Tweet this! Cognitive, affective, and behavioral health issues are now the costliest in the United States. Focusing on treatment and prevention strategies will require thoughtful health care payment and delivery reform: http://ow.ly/TuJn30jxrSr #investinkids #childrenshealthforum
Tweet this! Cognitive, affective, and behavioral health issues are now the costliest in the United States. Focusing on treatment and prevention strategies will require thoughtful health care payment and delivery reform: http://ow.ly/TuJn30jxrSr #investinkids #childrenshealthforum
![]() Tweet this! Health care systems already calculate the cost of healthcare over a patient’s lifetime to study health care delivery – what if they considered how much the prevention of an illness saves? http://ow.ly/TuJn30jxrSr #investinkids #childrenshealthforum
Tweet this! Health care systems already calculate the cost of healthcare over a patient’s lifetime to study health care delivery – what if they considered how much the prevention of an illness saves? http://ow.ly/TuJn30jxrSr #investinkids #childrenshealthforum
![]() Tweet this! Health systems do not operate in a vacuum in ensuring population health. Incentives should be shared with community organizations, child care, education, social services, and justice programs: http://ow.ly/TuJn30jxrSr #investinkids #childrenshealthforum
Tweet this! Health systems do not operate in a vacuum in ensuring population health. Incentives should be shared with community organizations, child care, education, social services, and justice programs: http://ow.ly/TuJn30jxrSr #investinkids #childrenshealthforum
Download the graphic below and share it on social media!
References
- McGinnis, J. M., D. M. Berwick, T. A. Daschle, A. Diaz, H. V. Fineberg, W. H. Frist, A. Gawande, N. Halfon, and R. Lavizzo-Mourey. 2016. Systems Strategies for Better Health Throughout the Life Course: A Vital Direction for Health and Health Care. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201609g
- Halfon, N., P. Long, D. I. Chang, J. Hester, M. Inkelas, and A. Rodgers. 2014. Applying a 3.0 transformation framework to guide large-scale health system reform. Health Affairs 33(11):2003-2011. https://doi.org/10.1377/hlthaff.2014.0485
- Halfon, N., P. H. Wise, and C. B. Forrest. 2014. The changing nature of children’s health development: New challenges require major policy solutions. Health Affairs 33(12):2116-2124. https://doi.org/10.1377/hlthaff.2014.0944
- Coordinated care organizations and public health authorities in collaboration. 2014. Oregon Health Authority. Available at: https://public.health.oregon.gov/ProviderPartnerResources/HealthSystemTransformation/Document/success-stories/case-study-lane.pdf (accessed May 2, 2017).
- Rogerson, B., R. Lindberg, M. Givens, and A. Wernham. 2014. A simplified framework for incorporating health into community development initiatives. Health Affairs 33(11):1939-1947. https://doi.org/10.1377/hlthaff.2014.0632
- Alley, D. E., C. N. Asomugha, P. H. Conway, and D. M. Sanghavi. 2016. Accountable health communities—addressing social needs through Medicare and Medicaid. The New England Journal of Medicine 374(1):8-11. https://doi.org/10.1056/NEJMp1512532
- Centers for Medicare and Medicaid Services. 2016. Medicare finalizes substantial improvements that focus on primary care, mental health, and diabetes prevention. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2016-Press-releases-items/2016-11-02.html (accessed May 2, 2017).
- Centers for Medicare and Medicaid Services. 2016. Vermont all-payer ACO model joins growing state-based efforts to deliver better health care, reduce costs. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2016-Press-releases-items/2016-10-26.html (accessed May 2, 2017).
- Busch, A. B., H. A. Huskamp, and J. M. McWilliams. 2016. Early efforts by Medicare accountable care organizations have limited effect on mental illness care and management. Health Affairs 35(7):1247-1256. https://doi.org/10.1377/hlthaff.2015.1669
- Roehrig, C. 2016. Mental disorders top the list of the most costly conditions in the United States: $201 billion. Health Affairs (35)6:1-6. https://doi.org/10.1377/hlthaff.2015.1659
- National Academies of Sciences, Engineering, and Medicine. 2015. Mental Disorders and Disabilities Among Low-Income Children. Washington, DC: The National Academies Press. https://doi.org/10.17226/21780
- National Research Council and Institute of Medicine. 2009. Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities. Washington, DC: The National Academies Press. https://doi.org/10.17226/12480
- MacArthur, G., R. Kipping, J. White, C. Chittleborough, R. Lingam, K. Pasch, D. Gunnell, M. Hickman, and R. Campbell. 2012. Individual-, family-, and school-level interventions for preventing multiple risk behaviours in individuals aged 8 to 25 years. The Cochrane Library. Available at: http://cochrane.org/CD009927/individual–family–and-school-level-interventions-for-preventing-multiple-risk-behaviours-in-individuals-aged-8-to-25-years (accessed May 2, 2017).
- Feinberg, M. E., M. Xia, G. M. Fosco, R. E. Heyman, and S. M. Chow. 2017. Dynamical systems modeling of couple interaction: A new method for assessing intervention impact across the transition to parenthood. Prevention Science 9:1-2. https://doi.org/10.1007/s11121-017-0803-3
- Mihalic, S. F., and D. S. Elliott. 2015. Evidence-based programs registry: Blueprints for healthy youth development. Evaluation and Program Planning 48:124-131. https://doi.org/10.1016/j.evalprogplan.2014.08.004
- Beardslee, W. R., P. L. Chien, and C. C. Bell. 2011. Prevention of mental disorders, substance abuse, and problem behaviors: A developmental perspective. Psychiatric Services 62(3):247-254. https://doi.org/10.1176/ps.62.3.pss6203_0247
- Molleda, L., M. Bahamon, S. M. S. George, T. Perrino, Y. Estrada, D. C. Herrera, H. Pantin, and G. Prado. 2017. Clinic personnel, facilitator, and parent perspectives of eHealth familias unidas in primary care. Journal of Pediatric Health Care 31(3):350-361. https://doi.org/10.1016/j.pedhc.2016.11.001
- Brown, C. H., D. C. Mohr, C. G. Gallo, C. Mader, L. A. Palinkas, G. Wingood, G. Prado, J. Poduska, R. D. Gibbons, S. G. Kellam, H. Pantin, J. McManus, M. Ogihara, T. Valente, F. Wulczyn, S. Czaja, G. Sutcliffe, J. Villamar, and C. Jacombs. 2013. A computational future for preventing HIV in minority communities: How advanced technology can improve implementation of effective programs. Journal of Acquired Immune Deficiency Syndromes 63(Supplement 1):S72-S84. https://doi.org/10.1097/QAI.0b013e31829372bd
- Love, S. M., M. R. Sanders, K. M. Turner, M. Maurange, T. Knott, R. Prinz, C. Metzler, and A. T. Ainsworth. 2016. Social media and gamification: Engaging vulnerable parents in an online evidence-based parenting program. Child Abuse and Neglect. 53:95-107. https://doi.org/10.1016/j.chiabu.2015.10.031
- Leslie, L. K., C. J. Mehus, J. D. Hawkins, T. Boat, M. A. McCabe, S. Barkin, E. C. Perrin, C. W. Metzler, G. Prado, V. F. Tait, and R. Brown. 2016. Primary health care: Potential home for family-focused preventive interventions. American Journal of Preventive Medicine 51(4):S106-118. https://doi.org/10.1016/j.amepre.2016.05.014
- Brown, C. H., A. Brincks, S. Huang, T. Perrino, G. Cruden, H. Pantin, G. Howe, J. F. Young, W. Beardslee, S. Montag, and I. Sandler. 2016. Two-year impact of prevention programs on adolescent depression: An integrative data analysis approach. Prevention Science 24:1-21. https://doi.org/10.1007/s11121-016-0737-1
- National Academies of Sciences, Engineering, and Medicine. 2016. Identifying Opportunities for Prevention and Intervention in the Youth Depression Cascade: Workshop in Brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/23397
- National Research Council and Institute of Medicine. 2009. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. Washington, DC: The National Academies Press. https://doi.org/10.17226/12565
- Health Care Payment and Learning Action Network. 2017. APM measurement: Progress of alternative payment models: LAN insights into APM action. Baltimore, MD: Centers for Medicare and Medicaid Services. Available at: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf (accessed May 2, 2017).
- Peterson, T. A., S. J. Bernstein, and D. A. Spahlinger. 2016. Population health: A new paradigm for medicine. The American Journal of the Medical Sciences 351(1):26-32. https://doi.org/10.1016/j.amjms.2015.10.011
- Institute of Medicine and National Research Council. 2015. Harvesting the Scientific Investment in Prevention Science to Promote Children’s Cognitive, Affective, and Behavioral Health: Workshop Summary. Washington, DC: The National Academies Press. https://doi.org/10.17226/18964
- Arthur, M. W., J. D. Hawkins, J. A. Pollard, R. F. Catalano, and A. J. Baglioni Jr. 2002. Measuring risk and protective factors for use, delinquency, and other adolescent problem behaviors: The Communities That Care Youth Survey. Evaluation Review 26(6):575-601. https://doi.org/10.1177/0193841X0202600601
- Sampson, R. J., S. W. Raudenbush, and F. Earls. 1997. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science 277(5328):918-924. https://doi.org/10.1126/science.277.5328.918
- Mistry, K. B., F. Chesley, K. LLanos, and D. Dougherty. 2014. Advancing children’s health care and outcomes through the Pediatric Quality Measures Program. Academic Pediatrics 14(5):S19-26. https://doi.org/10.1016/j.acap.2014.06.025
- National Research Council and Institute of Medicine. 2004. Children’s Health, the Nation’s Wealth: Assessing and Improving Child Health. Washington, DC: The National Academies Press. https://doi.org/10.17226/10886
- Masten, A., K. Burt, and J. D. Coatsworth. 2015. Competence and psycho-pathology in development. In Developmental psychopathology risk, disorder, and adaptation, edited by D. Cicchetti and D. Cohen. Hoboken, NJ: John Wiley and Sons. P. 704.
- Briggs, R. D., E. M. Stettler, E. J. Silver, R. D. Schrag, M. Nayak, S. Chinitz, and A. D. Racine. 2012. Social-emotional screening for infants and toddlers in primary care. Pediatrics 129(2):e377-384. https://doi.org/10.1542/peds.2010-2211
- The Aspen Institute. No date. Two-generation playbook. Washington, DC. Available at: http://b.3cdn.net/ascend/5e6780f32400661a50_pgm6b0dpr.pdf (accessed May 2, 2017).
- Dubowitz, H. 2014. The Safe Environment for Every Kid Model: Promotion of children’s health, development, and safety, and prevention of child neglect. Pediatric Annals 43(11):e271-277. Available at: https://njaap.org/wp-content/uploads/2020/01/SEEK-Peds-Annals-11-14.pdf (accessed September 1, 2020).
- National Academies of Sciences, Engineering, and Medicine. 2016. Parenting Matters: Supporting Parents of Children Ages 0-8. Washington, DC: The National Academies Press. https://doi.org/10.17226/21868
- Victorson, D., J. Manly, K. Wallner-Allen, N. Fox, C. Purnell, H. Hendrie, R. Havlik, M. Harniss, S. Magasi, H. Correia, and R. Gershon. 2013. Using the NIH Toolbox in special populations: Considerations for assessment of pediatric, geriatric, culturally diverse, non–English-speaking, and disabled individuals. Neurology 80(11 Supplement 3):S13-19. https://doi.org/10.1212/WNL.0b013e3182872e26
- DeWalt, D. A., H. E. Gross, D. S. Gipson, D. T. Selewski, E. M. DeWitt, C. D. Dampier, P. S. Hinds, I. C. Huang, D. Thissen, and J. W. Varni. 2015. PROMIS pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Quality of Life Research 24(9):2195-2208. https://doi.org/10.1007/s11136-015-0953-3
- Hamilton, C. M., L. C. Strader, J. G. Pratt, D. Maiese, T. Hendershot, R. K. Kwok, J. A. Hammond, W. Huggins, D. Jackman, H. Pan, and D. S. Nettles. 2011. The PhenX Toolkit: Get the most from your measures. American Journal of Epidemiology 174(3):253-260. https://doi.org/10.1093/aje/kwr193
- National Institute of Mental Health. n.d. Research domain criteria (RDoC). Bethesda, MD. Available at: https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml (accessed April 16, 2018).
- Institute of Medicine. 2015. Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: The National Academies Press. https://doi.org/10.17226/19402
- Cruden, G., K. Kelleher, S. Kellam, and C. H. Brown. 2016. Increasing the delivery of preventive health services in public education. American Journal of Preventive Medicine 51(4):S158-167. https://doi.org/10.1016/j.amepre.2016.07.002
- Gibbons, R. D., D. J. Weiss, E. Frank, and D. Kupfer. 2016. Computerized adaptive diagnosis and testing of mental health disorders. Annual Review of Clinical Psychology 12:83-104. https://doi.org/10.1146/annurev-clinpsy-021815-093634
- Torous, J., M. V. Kiang, J. Lorme, and J. P. Onnela. 2016. New tools for new research in psychiatry: A scalable and customizable platform to empower data driven smartphone research. JMIR Mental Health 3(2):e16. https://doi.org/10.2196/mental.5165
- Jones, D. E., M. Greenberg, and M. Crowley. 2015. Early social-emotional functioning and public health: The relationship between kindergarten social competence and future wellness. American Journal of Public Health 105(11):2283-2290. https://doi.org/10.2105/AJPH.2015.302630
- The Washington State Institute for Public Policy. 2017. Benefit-cost technical documentation. Olympia, WA. Available at: http://www.wsipp.wa.gov/TechnicalDocumentation/WsippBenefitCostTechnicalDocumentation.pdf (accessed May 2, 2017).
- Kemper, A. R., I. R. Mabry-Hernandez, and D. C. Grossman. 2016. US Preventive Services Task Force approach to child cognitive and behavioral health. American Journal of Preventive Medicine 51(4):S119-123. https://doi.org/10.1016/j.amepre.2016.05.016
- Tyler, E. T., R. L. Hulkower, and J. W. Kaminski. 2017. Behavioral health integration in pediatric primary care: Considerations and opportunities for policymakers, planners, and providers. New York: Milbank Memorial Fund. Available at: https://www.milbank.org/publications/behavioral-health-integration-in-pediatric-primary-care-considerations-and-opportunities-for-policymakers-planners-and-providers (accessed May 2, 2017).
- McCormick, E., S. E. Kerns, H. McPhillips, J. Wright, D. A. Christakis, and F. P. Rivara. 2014. Training pediatric residents to provide parent education: A randomized controlled trial. Academic Pediatrics 14(4):353-360. https://doi.org/10.1016/j.acap.2014.03.009
- Hawkins, J. D., S. Oesterle, E. C. Brown, R. D. Abbott, and R. F. Catalano. 2014. Youth problem behaviors 8 years after implementing the communities that care prevention system: A community-randomized trial. JAMA Pediatrics 168(2):122-129. https://doi.org/10.1001/jamapediatrics.2013.4009
- Oesterle, S., M. R. Kuklinski, J. D. Hawkins, M. L. Skinner, K. Guttmannova, and I. C. Rhew. 2018. Long-term effects of the Communities That Care trial on substance use, antisocial behavior, and violence through age 21 years. American Journal of Public Health. https://doi.org/10.2105/AJPH.2018.304320
- Tolan, P. H., V. McBride Murry, A. Diaz, and R. Seidel. 2016. Life Span and Legal/Policy Research as Dual Focuses for Identifying and Implementing Opportunities to Realize Health Equity. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201610c