Realizing the Full Potential of Precision Medicine in Health and Health Care: A Vital Direction for Health and Health Care
 This publication is part of the National Academy of Medicine’s Vital Directions for Health and Health Care Initiative, which commissioned expert papers on 19 priority focus areas for U.S. health policy by more than 100 leading researchers, scientists, and policy makers from across the United States. The views presented in this publication and others in the series are those of the authors and do not represent formal consensus positions of the NAM, the National Academies of Sciences, Engineering, and Medicine, or the authors’ organizations.
Learn more: nam.edu/VitalDirections
This publication is part of the National Academy of Medicine’s Vital Directions for Health and Health Care Initiative, which commissioned expert papers on 19 priority focus areas for U.S. health policy by more than 100 leading researchers, scientists, and policy makers from across the United States. The views presented in this publication and others in the series are those of the authors and do not represent formal consensus positions of the NAM, the National Academies of Sciences, Engineering, and Medicine, or the authors’ organizations.
Learn more: nam.edu/VitalDirections
Introduction
Major achievements in scientific research have enabled a new era of health care delivery and treatment. Understanding of the underlying mechanisms of diseases is increasing and allowing scientists to develop new drugs, targeted therapies, and preventive strategies. A new form of health care that is based on data, algorithms, and precision molecular tools has become possible. Precision medicine—an emerging approach that integrates investigation of mechanisms of disease with prevention, treatment, and cure, resolved at the level of the individual subject or patient—has great potential to contribute to solutions for providing high value health care by improving outcomes while decreasing cost. Despite recent breakthroughs and the growing momentum behind precision medicine, as evidenced by the launch of the US Precision Medicine Initiative, there remain substantial challenges and barriers to its broad implementation in medical practice, including generating the needed evidentiary support for precision medicine, addressing data-sharing and infrastructure needs, incorporating genomic information into clinical care and research, reconciling the economics of precision medicine, and securing participant engagement and trust. Policy makers will need to address those critical challenges if the full potential of precision medicine is to be realized. Building on the input of national leaders in precision medicine, this paper identifies and explores the challenges to and opportunities to achieve precision medicine and offers specific recommendations to achieve its potential.
Overview and State of Science
Precision medicine is a bold concept that captures and integrates the endeavors and the outcomes of research, health, and health care. The ability to tailor prevention, diagnostics, and therapeutics to individual patients is at the heart of precision medicine. Central to that effort is the ability to assemble a fuller understanding of a patient’s health, to share that information (securely) with researchers who are looking for more effective health advice and therapies, and to transition relevant findings back to patients and their providers to improve health outcomes. The aspirations and challenges of precision medicine, as defined and enunciated in the 2011 National Research Council report Toward Precision Medicine: Building a Knowledge Network for Biomedical Research (NRC, 2011), encompass—indeed reach beyond—our entire biomedical research, health, and health care enterprise. In the precision medicine ecosystem, physical and natural scientists and engineers virtually merge their concepts and tools, and they engage with clinicians and social, behavioral, and population investigators to produce and share a computational “learning system”—a knowledge network that aggregates, integrates, accesses and analyzes information from large patient cohorts, healthy populations, and experimental systems and organisms—to reveal new laboratory-testable hypotheses, to classify diseases by mechanisms, and to provide precise prevention, diagnosis, and treatment options for each person.
Precision medicine, especially as a national or international enterprise, is an audacious aspiration. Consider, however, the potential effects of this endeavor: we would integrate into an iteratively developing knowledge network a working understanding of the logic and mechanisms of biologic processes, thereby contributing continuously and in real time to evidence-based prevention of, treatment for, and cure of chronic, infectious, and rare diseases. Deeper understanding of disease mechanisms would cut drug-development costs by enabling smaller, faster, and more successful clinical trials (as in the case of the approval of Herceptin for breast cancer); reduce the use of prescriptions that are ineffective or produce adverse outcomes (as in the case of Abacvir for HIV); and limit clinical tests that are uninformative for individuals or groups of individuals (as is the case for diagnostic sequencing for rare diseases). Collection and use of data on diverse populations would facilitate and motivate democratization of public health and tailor it to individuals. All those elements working together would yield a healthier, more productive population and drive an overall decline in the slope of the health care cost curve.
We have made great strides in our capacity to initiate the virtuous circle of data collection to health advice and recommended therapies. However, economic, regulatory, social, and technical barriers and challenges must be resolved before the precision medicine ecosystem can realize its full potential. In creating a more supportive policy environment to overcome the challenges, policy makers will need to work alongside key stakeholder groups academe, private industry, government, health care providers, patients, and the general public—to engage in more coordinated efforts to establish precision medicine as the driver of our health and health care system.
To empower scientific research and catalyze future innovations in health care, President Obama established a US research program to accelerate progress in the implementation of precision medicine. In his 2015 State of the Union Address, the president announced the launch of the Precision Medicine Initiative (PMI) “to bring us closer to curing diseases like cancer and diabetes, and to give all of us access to the personalized information we need to keep ourselves and our families healthier.” Ten days later, the president detailed his vision for the initiative at a White House event, placing patients at the center of its design and charging the National Institutes of Health (NIH) with leading a PMI Cohort Program (PMI-CP) and a companion cancer component. The PMI-CP is a monumental and critical effort that offers many promising research opportunities for achieving better health and health care. Given its cutting-edge nature, the PMI-CP will undoubtedly face a number of challenges (Box 1). To translate precision medicine into health care, the next administration should consider policies that will facilitate the reduction and eventual elimination of those challenges so that precision medicine can be translated into health care. Critical directions for the advancement of precision medicine include reducing gaps in access to and availability of essential data; establishing data-sharing platforms, incentives, and infrastructure; promoting the use of genomic information in clinical care; creating better economic models for precision medicine; and converting a skeptical patient population to one that is committed and engaged.
Key Issues, Barriers to, and Opportunities for Progress
Evidence Generation
Limited evidence that precision medicine improves clinical outcomes, increases cost-effectiveness and affordability, and improves quality of care presents a major barrier to its adoption. Before precision medicine can be broadly implemented, there is a need to develop a robust evidentiary foundation of its value. Randomized controlled trials (RCTs) are the gold standard for evidence generation; however, an emerging approach is for health care organizations to collect data as part of continuing clinical care as a means of generating evidence (Ginsburg, 2014) on patient and economic outcomes. Although much research is still needed to demonstrate the validity of that method, it holds promise as an approach to address the surging numbers of genomic discoveries, increasing trial costs, and low margins for diagnostic products. As learning health-system models emerge to facilitate evidence creation, the questions become, When should RCTs be required? When they are, can they include uniform evidentiary standards and minimum requirements for socioeconomic diversity in addition to the relevant outcomes required for reimbursement reviews and evaluations among payers and regulatory agencies?
The evolving nature of evidence generation for precision medicine will necessitate agreement between stakeholders and policy makers on standards for initial clinical use and on mechanisms for postmarketing collection of data to refine the precision-medicine evidence base. Once a consensus is reached on standards for initial clinical use, regulatory and reimbursement policy can be updated to reflect such standards, contingent on mandatory postmarketing data collection that is required for final approval.
In building the evidence base for precision medicine, postmarket data on diverse populations will have to be collected continuously. Evidence demonstrating the potential of precision medicine to improve care quality and cost-effectiveness will take time to develop, and mechanisms must be put into place to ensure continuing evidence generation and assessment. As recommended in the 2016 Institute of Medicine report, Biomarker Tests for Molecularly Targeted Therapies: Key to Unlocking Precision Medicine (IOM, 2016) the development of a rapid learning system for biomarker tests for molecularly targeted therapies would be essential to facilitate knowledge generation, and continuous learning and accelerate the translation of lessons learned into better patient care and improved clinical outcomes. The recommended actions focus on improving the policy environment, data infrastructure and patient care processes related to biomarker tests for molecularly targeted therapies.
Thoughtful consideration of study designs that are based on the available postmarketing data that support the continued use of various precision-medicine approaches is critical. Precision-medicine evidence generation could be tied to an adaptive approach or pathway for treatment so as to ensure that patients who have unmet needs have access to promising therapies as they become available.
Furthermore, as precision medicine expands into routine clinical use, the evidence supporting its use must come from a broad cross-section of the population. Thus, there is a need to develop and implement a series of criteria to indicate when the use of RCTs in precision medicine is required for evidence generation, including uniform evidentiary standards, the minimum requirements for socioeconomic diversity in clinical-trial enrollment, and the relevant outcomes required for reimbursement reviews and evaluations among payers and regulatory agencies. As recommended in the IOM (2016) report, HHS should facilitate a process for the development of common evidentiary standards of clinical utility for biomarker tests for molecularly targeted therapies by convening one or more independent, public-private multi-stakeholder bodies. These common evidentiary standards would inform the development of an integrated review process for coordinated regulatory, coverage and reimbursement decisions.
To generate the necessary evidence to assess the health-economic impact of precision-medicine technologies, final regulatory and payer approval could be made contingent on the inclusion of a health-economic impact analysis. Acknowledging that such data, especially longitudinal data, would be difficult to generate within the timeframe of a clinical trial, other approaches could be used to project long-term economic effects, including economic modeling.
Priority considerations for enhancing evidence generation and use include
- Developing and adopting an evidence framework to guide clinical implementation, and then ensure continuing evidence generation, especially in the postmarketing–postapproval setting, for precision medicine tests and therapies.
- Developing and adopting a flexible framework (that ties evidence to the use case) to balance the use of RCTs against “big data” and observational analysis in precision medicine.
- Including health-economic impact analysis (encompassing cost effectiveness and long-term savings) for regulatory and payer approval.
Data Sharing and Infrastructure Needs
The path to precision medicine requires access to large-scale, detailed, and highly integrated patient data to advance our understanding of the genomic, molecular, phenotypic, clinical, and digital signatures of disease. Precision medicine requires not only big data but diverse data. Advancing the field and improving understanding of the complexities of human health and disease will require aligning often-unstructured datasets into a comprehensive knowledge network (NRC, 2011). The vast majority of repositories of research and clinical data cannot now be easily combined with one another. Furthermore, in drug development, pharmaceutical companies could take advantage of clinical trials to explore, learn about, and generate additional hypotheses for collecting data. All too often, clinical trials focus on testing a primary hypothesis and—for reasons related to cost, time, and fear of the unknown—fail to incorporate exploratory genomic, digital, and other measures to help to create the learning necessary to drive precision medicine. As recommended in the 2015 Institute of Medicine report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk, some companies have begun to place clinical trial data in the public domain; however, data on product failures that would be highly valuable for scientific inquiry are not shared.
Recent progressive policies have facilitated notable progress. At the launch event for the PMI in January 2015, the Obama Administration emphasized that patients should “have access to their own health data—and to the applications and services that can safely and accurately analyze it” (The White House, 2015). The administration followed through by regulating electronic health records (EHRs) to offer data access to patient designated apps and requiring the use of such technology by providers in the Medicare and Medicaid payment incentive programs by 2018. The Office of Civil Rights of the Department of Health and Human Services further clarified that patients have a digital right to access data protected by the Health Insurance Portability and Accountability Act (HIPAA) and can direct it to an end point of their choosing at a marginal cost that can cover only the production of the digital copy (in other words, near zero). Leading health information technology (HIT) vendors publicly pledged to support a “sync for science” program by upgrading their technology in a manner that enables a patient to connect a precision medicine direct enrollment application to health data otherwise available only via a patient portal.
Considering those developments, we are entering 2017 seemingly far closer to Mitchell Kapor’s vision of a “health Internet” (Kapor, 2009), an open platform that would connect providers, payers, and consumers to a growing number of applications, including ones designed for precision medicine. New datasets not traditionally considered part of a health record—digital assessment, intent, and monitoring data—would be available for patients to connect seamlessly via the health Internet to connected, trusted databases that store, analyze, and trigger therapeutic and preventive recommendations. In combination with the Obama Administration’s unprecedented efforts to open up health data and focus on aligning payment with improved outcomes, we ought to be entering 2017 with ingredients that enable data-driven health care delivery models that foster better care for both individuals and communities. To get there, the next administration will need to tackle key challenges that have hindered progress despite unprecedented (often bipartisan) policy commitment. The challenges are discussed below.
The Economics of Health-Data Production
The core of a provider’s health care dataset is the complete, digitized longitudinal clinical and administrative records designed in part to meet the needs of payers. Through the Patient Protection and Affordable Care Act, public payers have accelerated a transition from requiring data solely for the purpose of documenting an encounter to measuring outcomes and facilitating better care coordination. That payer-driven demand has sharpened provider focus on the types of health information collected and shared to maximize reimbursement. Previously unstructured data buried in clinical notes, such as smoking status, are now accessible in a form that allows search queries to generate lists of patients who have gaps in care that should be addressed.
Despite wide agreement among public and private payers to drive more outcomes-based payment models, most provider revenue today remains tethered to the fee-for-service model. Thus, the adoption and use of advanced HIT systems capable of performing such queries or publishing such structured data remain challenging. Assuming that precision medicine will achieve better outcomes, any effort to maximize the flow of data for precision medicine should begin by accelerating a shift toward pay-for-value models. That shift would depend on HIT systems to store, share, and provide the very data that support precision medicine.
The Regulation of Data Access, Privacy, and Security
Since the 2009 passage of the Health Information Technology for Economic and Clinical Health Act, we have been on a regulatory “escalator” that has equipped providers with more powerful HIT. But that journey is not yet complete. Under both the “meaningful use” and “advancing care information” Centers for Medicare & Medicaid Services (CMS) incentive programs, providers must use certified technology, but three policy levers deserve further attention if technology is to be aligned with precision-medicine requirements.
First, the Common Clinical Data Set (CCDS) needs to be broadened. Health-records vendors compete, in part, on the value of their underlying data models that can be put to use in achieving a provider’s operational needs; to facilitate interoperability among vendors, the administration regulates the transformation of a set of patient data into a more open machine-readable format that is free of intellectual property (IP) restrictions. An important consideration for the next administration will be the ability of precision-medicine researchers to provide a feedback loop on any necessary expansions or adjustments of the CCDS.
Second, application programming interface (API) access needs to be standardized and expanded. Health records vendors manage the business and technical models associated with providing third-party applications access to the CCDS via an API, a contract that explains how to access data and logic securely while protecting patient privacy; current policy compels EHR vendors to open developer access to enable connectivity to an app of a patient’s choice but leaves open the methods and economic terms. An important consideration will be standardization of those methods among vendors and expansion of patient API access among all regulated HIT systems, including medical devices regulated by the Food and Drug Administration (FDA) and other certified EHR modules.
Third, oversight needs to be strengthened. Two enforcement methods are available to ensure that certified technology delivers information access as tested: surveillance and testing in the field and use of emerging information blocking tools, including Office of the Inspector General enforcement of antikickback waivers for health systems that subsidize EHR adoption. The next administration should consider more aggressive surveillance to ensure that patients and providers who have application access can query a complete CCDS and that nontechnical barriers, such as cost and burden, are minimized.
Encouraging Further Voluntary Industry Consensus on Standards
Since the Clinton Administration, it has been US policy to leave technical-standards development to voluntary, industry-led bodies that are free to innovate and adjust without undue government interference. In January 2012, the Obama Administration clarified that in some elements of national importance, the government can play a convening role to spur technologic advances in fields that need it and encourage wider adoption (OSTP, 2011). For precision medicine to succeed, adoption of voluntary industry standards will be necessary to ensure that data can be synchronized around the patient, not around the institution that treated the patient for a particular episode or delivered services over the course of an insurance enrollment cycle. If we make the necessary policy adjustments and encourage industry adoption of standards, we will deliver on the promise of using robust and broadly available digital data to discover more appropriate therapies for each individual patient and to ensure that the therapies are delivered via care models that reward delivering better outcomes.
Priority considerations for enhancing evidence generation and use include
- The administration broadening the CCDS to facilitate interoperability among vendors, to create a more open machine-readable format that is free of IP restrictions, and to ensure that patients who have application access can query a complete CCDS with only minimal nontechnical barriers, such as cost and burden.
- The administration standardizing methods among vendors and expanding patient API access among all regulated HIT systems, including FDA-regulated medical devices and other certified EHR modules.
- NIH and health systems driving adoption of voluntary industry standards for structured data or common data elements to be captured in EMRs for synchronizing data around the patient, not around the institution that treated the patient for a particular episode or that delivered services over the course of an insurance enrollment cycle.
- The digital health community (EMR vendors, health systems, and app developers) working toward strengthening digital identities and standardizing how securely identified patients authorize the sharing of health information for precision medicine.
- Strengthening consumer protections related to the use (or misuse) of data via patient-designated apps not subject to HIPAA regulation by encouraging industry adoption of a model code of conduct akin to a “digital Hippocratic oath” that can be enforceable by such agencies as the Federal Trade Commission.
- Policies promoting incentives to share data among all stakeholders.
- Interagency funding mechanisms (for example, shared by NIH, the Department of Energy, the Defense Advanced Research Projects Agency, and the National Institute of Standards and Technology) to motivate cross-sector partnerships (.edu, .gov, .com, and .org) that harness intellectual synergies and enable sustainability of infrastructure and activities necessary to realize the full potential of a public benefit from federal investment
in precision medicine for biomedical research, public health, and health care.
Integrating Genomic and Other Molecular Data into Clinical Care and Research
Moving genomic and other molecular information into routine health care delivery is critical for a precision medicine–powered health system. Genomic information is not widely used in clinical care. Some medical centers are incorporating it into clinical research, including the University of Michigan cancer sequencing program (MI-ONCOSEQ), the Geisinger Medicine Institute’s MyCode Community Health Initiative in partnership with Regeneron Pharmaceuticals, Inova Translational Medicine Institute, and several research programs supported by the National Human Genome Research Institute, such as Implementing Genomics in Practice (IGNITE), Electronic Medical Records and Genomics Network (eMERGE), and Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT). NIH has also developed the Clinical Genomic Resource (ClinGen) for sharing information about genomic variants and phenotypes and ClinVar, which houses data on evidence of variants of clinical significance associated with disease. The National Cancer Institute (NCI) has launched research programs aimed at identifying the genetic drivers of specific cancers with a goal for developing targeted therapeutics: the Cancer Genome Characterization Initiative, the Cancer Genome Atlas (TCGA), and Therapeutically Applicable Research to Generate Effective Treatments (TARGET). Their data are housed in the newly announced NCI Genomic Data Commons. Together, however, the use of genomic information has been used largely for specific applications such as diagnosis for severely sick newborns, developmental delay, or unidentified genetic disorders—and to target treatments for some cancers.
In particular, how we think about the genomes of individuals presents a major barrier to the use of genomic information in health care. Medical-genetics experts have promoted thinking of genetic and genomic information as “exceptional” and the idea that it requires great protections. For precision medicine to succeed, we need a fundamental change in medical thinking to move genomics from exceptional to routine information for the understanding of health and health care—to consider a patient’s genome sequence as foundational information for health care, just as we consider blood pressure, pulse, temperature, heart rate, height, and weight. We will continue to learn more about the role of genomic variation in health. The American College of Medical Genetics and Genomics has defined 56 genes that have been established as relevant to medical conditions and recommends that variants in these genes be reported whenever clinical exome or genome sequencing is done (Green et al., 2013). As the evidence of the clinical utility of genomic information increases, we need to change how we think about the incorporation and use of this health care information.
A laudable future state of health care would include the effective implementation of precision medicine into learning health systems—health systems that implement genomics, gather data, analyze the data, and then use the results to change care paradigms (IOM, 2015). Genome sequences would be available for most patients and would be integrated into annual testing with other novel platforms (such as metabolic or immune profiling) and into patient-reported information to predict early onset of common and expensive diseases and thus allow prevention or early detection and intervention. All molecular, health care, and patient-reported information would be monitored to identify patterns and outliers, which would be investigated to improve our understanding of health care and health care delivery effectiveness.
Beyond the fundamental issue of changing how we view and use genomic information, key issues for implementation of genomics and its integration into health care include the need to generate additional evidence that demonstrates the clinical usefulness of genomics, the availability and quality of genome interpretation tools, understanding of how to use genomic information in clinical care, knowing whether genomics will decrease or increase the overall cost of health care and improve outcomes, and public concerns about the potential misuse of genomic information.
Priority considerations for enhancing evidence generation and use include
- NIH and other agencies engaging communities of stakeholders—diverse participants and providers—in supporting the development of tools and educational resources to promote the integration of precision-medicine information on diverse populations into clinical-practice settings.
- FDA and other regulatory agencies seeking novel pathways to develop and deploy innovative mechanisms to oversee the rapid translation of research findings from precision medicine to health care delivery.
- NIH supporting the development of a national genomic-variant database, which would provide an interpretive resource for precision medicine.
- Medical-education oversight bodies, such as the Liaison Committee on Medical Education and the American Board of Medical Specialties, incorporating genomic-education requirements into medical education, residency and fellowship training, continuing-medical-education requirements, and maintenance of certification examinations to ensure a trained workforce and transition to the foundational nature of genomic information for health and health care.
- NIH engaging in collaborations to support the development of a centralized global resource (a toolbox) for the effective integration and use of genomic information in health information systems.
- NIH encouraging the development and dissemination of knowledge about precision medicine by ensuring sufficient resources and funding support for implementation, dissemination, and outcomes research (so-called T3 and T4 domains of translational research) and public and provider education.
- Congress expanding the Genetic Information Nondiscrimination Act of 2008 to provide full protection against misuse of genomic information for any purpose.
Innovation in Diagnostics, Drug Discovery, and the Economics of Precision Medicine
The challenge in developing new medicines has never been greater than it is today. The aggregate cost of developing a new medicine, in light of attrition rates, has been estimated at $2.7 billion (DiMasi et al., 2016). Extensive investment in research and the large cost of failure, particularly in development, have been used to explain the rising cost of drug discovery. Many reimbursement failures occur because therapies are not substantially different from established medicines; in many other cases, therapies offer benefits to patients but their value cannot be demonstrated experimentally through the comparator studies mandated by health-technology assessment agencies. Precision medicine aims to change the economics of drug and diagnostic development through a deeper understanding of the mechanisms of disease driven by the computational-knowledge network that continuously merges biomedical, clinical, and social and behavioral information. The new knowledge will increase the efficiencies of product development and approval.
Beyond novel targeted therapies, some of precision medicine’s greatest benefits may lie in identifying healthy people who are at high risk for disease and for whom efficacious therapies exist. The value to society is twofold: avoiding unnecessary treatment and identifying patients who otherwise would not be treated (Goldman et al., 2013). Depending on the disease, a precision-medicine innovation—one that more accurately identifies people who are at risk for the disease and is coupled to an intervention that reduces incidence even by as little as 10%—could generate hundreds of billions of dollars in value in the form of longer, healthier lives enjoyed by the US population (Dzau et al., 2015). The potentially large value generated by personalized diagnostic tests raises the question of why these diagnostic tests have not flourished as rapidly as expected (Aspinall and Hamermesh, 2007).
New diagnostics do not emerge in a vacuum. Rather, they result from investment of capital to finance research and development. The potentially large difference between the value generated by precision diagnostics and the price that they command raises the question of whether innovators have sufficient incentives. That question is particularly relevant for diagnostic tests that are not linked to targeted therapies. Manufacturers of targeted cancer therapies recoup the cost generated by a diagnostic test by charging prices that exceed the costs of production (Yin et al., 2012). With more effective targeting of treatment, the value of a therapy increases. Thus, as long as price and revenue are related to value, there are incentives to develop a companion diagnostic for a targeted therapy.
More broadly, the current reimbursement environment does not reward innovators for the value created by their tests. The development of a precision medicine-based diagnostic test by one company does not preclude development by others, and this limits economic incentives for test development. Moreover, reimbursement for diagnostics is typically cost-based rather than value-based. Third-party payers in the United States largely follow the standards set by CMS, which pays for diagnostic tests according to its clinical laboratory fee schedule. Reimbursement for diagnostic tests is therefore based on Common Procedural Terminology (CPT) codes, which historically set prices according to specific procedures conducted during a test (such as extraction and amplification of DNA). To determine the total price of a diagnostic test, codes for individual procedures were “stacked”; this method decouples price from value. The experience of Oncotype DX® is instructive. Oncotype DX predicts both the recurrence of breast cancer and the likelihood that a patient who has early-stage breast cancer will benefit from chemotherapy. Genomic Health, the manufacturer of Oncotype DX, used a miscellaneous CPT code rather than stacking codes to price its test. Meanwhile, the firm commissioned clinical and health-economic studies to demonstrate the value of its test and, over the course of several years, had obtained nearly complete payer coverage (Gustavsen et al., 2010). Genomic Health also entered into risk-sharing agreements with payers to secure its market price. Although new molecular-pathology codes are replacing older stacked codes, reimbursement is still not systematically related to value and potential downstream cost savings.
The rules for diagnostics stand in marked contrast with reimbursement for novel therapies, which are increasingly reimbursed on the basis of the value—measured according to quality-adjusted life years—that they generate. Ironically, diagnostic tests influence an estimated 60–70% of all treatment decisions but account for only 5% of hospital costs and 2% of Medicare expenditures; this suggests an imbalance between the value that the diagnostic tests generate and the amount of reimbursement for them (The Lewin Group, Inc., 2005).
Reconciling concerns about rising costs and improving health associated with medical technologies is central to the debate over advancing precision medicine; that is, how can we design reimbursement and regulatory incentives to encourage “valuable” innovation?
Priority considerations for enhancing evidence generation and use include
- NIH supporting research to assess the value of genomic testing and similar diagnostics in the context of the total cost of lifetime health care and to improve the quality of life and patient outcomes for individuals and populations.
- CMS and the payer community considering that the advancement of precision medicine represents an era of payment reform and that payment reform shifting toward value should incorporate genomic tests.
- The administration taking up patent reform in connection with precision medicine to provide exclusivity for companies that are willing to assume risk in developing precision-medicine approaches.
- FDA, CMS, NIH, and the Agency for Healthcare Research and Quality developing, adopting, and coordinating an innovative evidence-generation strategy that uses coverage with evidence development, uses data captured in the postmarketing and postapproval setting for precision-medicine tests and therapies, and includes health-economic effect analysis (encompassing cost-effectiveness and long-term savings) for regulatory and payer
approval.
Participant Engagement and Trust
Like no biomedical science ever before, precision medicine requires participant engagement. For discovery in precision medicine to be accelerated and services based on it to be adopted, people—not just patients—must be involved. That is because, although it is important for some aspects of the science to have data on individuals in the clinical context, it is also important to understand the continuum of health and disease on the basis of data on the experience of many people in diverse communities. Data from self-tracking devices and on the built environment and other non-clinical aspects of people’s lives will help to complete the picture essential for precision medicine.
There is a long way to go before participants have a substantive role in precision medicine. In part, the place of participant engagement is not yet accepted because there is no substantial evidence base for its inclusion. That situation is not unlike other aspects of precision medicine, but it has special challenges. Many funders, payers, researchers, and clinicians equate engagement with simple recruitment and retention, but it means much more in this context. It requires relationships with individuals and communities. It requires trust—not only that the people invited to participate should offer trust but that the organizations and entities involved in precision medicine should demonstrate trustworthiness.
There are important resource implications in authentic participant engagement that is based on trust. Trustworthy systems should be transparent and open. Transparency—from simple openness about possibilities, probabilities, and honest presentation of limitations to open science—is expensive. There are no ready-built systems for these activities; they have not been considered essential for research. It is possible that new economies will emerge, but they are not yet apparent. Today’s researchers are more likely to use time and funding for other aspects of research and not for recruitment of participants. Further along the continuum, in the realm of the implementation of precision medicine, clinicians involved in gathering data in a learning health care system or implementing new guidelines that result from precision medicine do not have the resources to seek patient-centered outcome reports or to involve patients in decision making—a hallmark of true engagement. Yet there is reason for optimism that these challenges can be overcome through participants’ embrace of social media, the Internet, and patient-advocacy groups and their increasing engagement in the conversation on privacy.
Authentic engagement, embedded in trust, must overcome a number of barriers. Participants range in their preferences for engagement from deeply engaged in determining the most relevant questions to not wanting to participate at all. And although there is growing pressure to engage consumers—coming largely from citizen-scientists, community-based participatory researchers, and such nascent entities as the Patient-Centered Outcomes Research Institute—researchers and clinicians have little experience in thinking of participants as partners in these endeavors. Only when the effect of engagement has a solid evidence base will it be supported and even promoted. It will also be critical to increase the literacy of the public in using more accessible methods than are currently imagined or deployed. For needed progress to occur, efforts to advance precision medicine must simultaneously increase the trust felt by the patient population and build the evidence base on the value of authentic engagement.
Priority considerations for enhancing evidence generation and use include
- NIH working with communities and research networks to establish best practices for engagement of participants and to determine metrics for trustworthiness and participation.
- NIH, FDA, and other agencies piloting open science to make scientific research, data, and dissemination accessible to all levels of society.
- Developing educational programs beginning at early educational stages to ensure genomic literacy, emphasizing precision medicine and related concepts, including the benefits of data sharing.
Summary Recommendations for Vital Directions
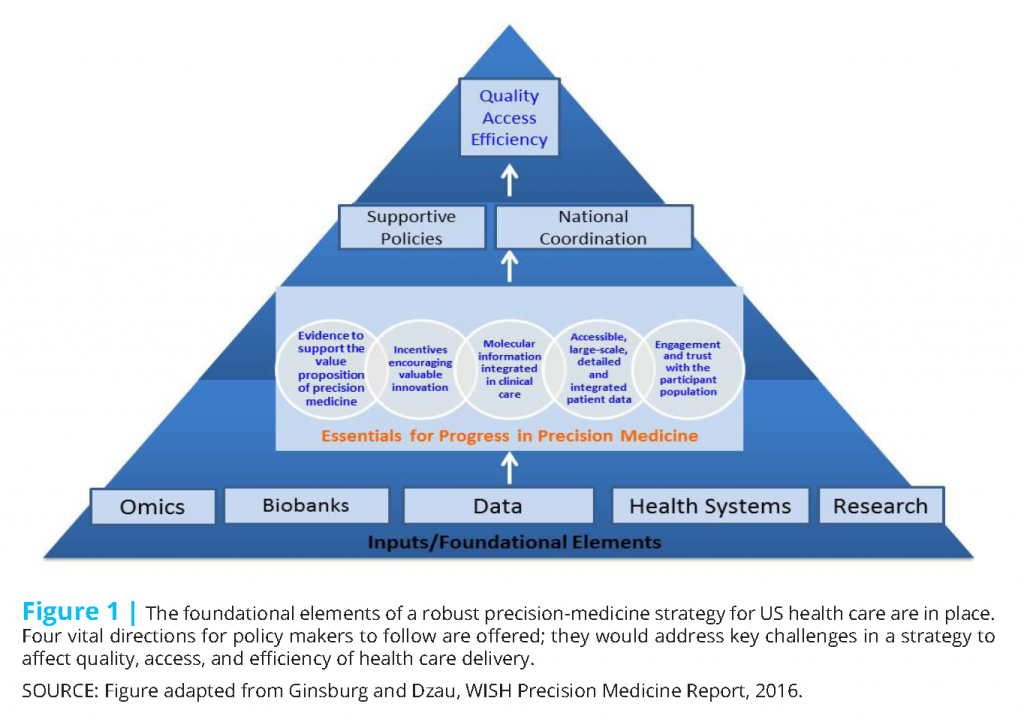
Precision medicine is an audacious but necessary aspiration if we are to achieve a health care delivery system that can provide accessible, high-quality, and efficient health care. To move the nation toward achieving the promise of precision medicine, we identify five vital directions (Figure 1) for the next administration’s consideration.
- Develop evidence of precision medicine’s effect. Provider and patient adoption and regulatory approval of and reimbursement for precision medicine requires a robust evidentiary framework for evaluation of its effect on outcomes.
- Accelerate clinical data integration and assessment. Advancing precision medicine and achieving a greater understanding of the complexities of human health and disease will require aligning and integrating diverse, often unstructured datasets into a comprehensive knowledge network.
- Promote integration of molecular guidance into care. Moving genomic and other information into routine health care delivery will be critical for integrating precision medicine into health systems. We need to adopt an approach to genetic and genomic information that considers a patient’s genome sequence as foundational information for health care.
- Develop innovation-oriented reimbursement and regulatory frameworks. The current reimbursement environment does not reward innovators for the value created by their diagnostic tests. Rather than being value-based, reimbursement for diagnostics is typically cost-based and discourages the translation of innovative tests and therapies. Incentives to develop the evidence base and the economic model that support precision medicine will be crucial.
- Strengthen engagement and trust of the public. Participant engagement is essential for discovery in precision medicine to be accelerated and for services based on precision medicine to be adopted. Effective engagement will necessitate relationships with individuals and their communities and attaining their trust to overcome existing barriers.
For those vital directions to be followed successfully, the United States will need a coordinated, collaborative effort that brings together the key stakeholders from the public, private, academic, and government sectors. That will require orchestration by a nonconflicted neutral convener. The convener would be charged with coordinating the development of a precision-medicine policy agenda to support the development, implementation, and integration of key precision-medicine infrastructure, data architecture, and tools into health care delivery.
References
- Aspinall, M. G., and R. G. Hamermesh. 2007. Realizing the promise of personalized medicine. Harvard Business Review 85(10):108-117. Available at: https://hbr.org/2007/10/realizing-the-promise-of-personalized-medicine (accessed July 28, 2020).
- DiMasi, J. A., H.G. Grabowski, and R.W. Hansen. 2016. Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics 47:20-33. https://doi.org/10.1016/j.jhealeco.2016.01.012
- Dzau, V. J., G.S. Ginsburg, K. Van Nuys, D. Agus, and D. Goldman. 2015. Aligning incentives to fulfill the promise of personalised medicine. Lancet 385(9982):2118-2119. https://doi.org/10.1016/S0140-6736(15)60722-X
- Ginsburg, G. 2014. Medical genomics: Gather and use genetic information in health care. Nature 508(7497): 451-453. https://doi.org/10.1038/508451a
- Goldman, D., C. Gupta, E. Vasudeva, K. Trakas, R. Riley, D.N. Lakdawalla, D. Agus, N. Sood, A.B. Jena, and T. Philipson. 2013. The value of diagnostic testing in personalized medicine. Forum for Health Economics & Policy 16(2):121-133. Available at: https://www.rand.org/pubs/external_publications/EP51572.html (accessed July 28, 2020).
- Green, R. C., J.S. Berg, W.W. Grody, S.S. Kalia, B.R. Korf, C.L. Martin, A.L. McGuire, R.L. Nussbaum, J.M. O’Daniel, K.E. Ormond, H.L. Rehm, M.S. Watson, M.S. Williams, and L.G. Biesecker. 2013. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine 15(7):565-574. https://doi.org/10.1038/gim.2013.73
- Gustavsen, G., K. Phillips, and K. Pothier. 2010. The reimbursement landscape for novel diagnostics. Weston, MA: Health Advances. Available at: https://www.bio.org/sites/default/files/legacy/bioorg/docs/Health_Advances&BIO_Novel_Diagnostics_Reimburs_20110103.pdf (accessed July 28, 2020).
- Institute of Medicine. 2015. Genomics-Enabled Learning Health Care Systems: Gathering and Using Genomic Information to Improve Patient Care and Research: Workshop Summary. Washington, DC: The National Academies Press. https://doi.org/10.17226/21707
- National Academies of Sciences, Engineering, and Medicine. 2016. Biomarker Tests for Molecularly Targeted Therapies: Key to Unlocking Precision Medicine. Washington, DC: The National Academies Press. https://doi.org/10.17226/21860
- Kapor, M. 2009. Building the Health Internet. Presented at The Meeting at Harvard on a Health Information Technology Platform, Boston, MA.
- The Lewin Group, Inc. 2005. The Value of Diagnostics Innovation, Adoption and Diffusion into Health Care. Available at: https://dx.advamed.org/sites/dx.advamed.org/files/resource/Lewin%20Value%20of%20Diagnostics%20Report.pdf (accessed July 28, 2020).
- NHGRI (National Human Genome Research Institute). 2015. Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT). Available at: https://www.genome.gov/27558493/newborn-sequencingin-genomic-medicine-and-public-health-nsight/ (accessed June 14, 2016).
- NHGRI. 2016. Implementing Genomics in Practice (IGNITE). Available at: https://www.genome.gov/27554264/implementing-genomics-in-practiceignite/ (accessed June 14, 2016).
- NIH (National Institutes of Health). 2016. About the Precision Medicine Initiative Co¬hort Program. Available at: https://www.nih.gov/precision-med¬icineinitiative-cohort-program (accessed June 14, 2016).
- National Research Council. 2011. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: The National Academies Press. https://doi.org/10.17226/13284
- OSTP (Office of Science and Technology Policy). 2011. A strategy for American innovation—securing our economic growth and prosperity. Washington, DC: The White House. Available at: https://obamawhitehouse.archives.gov/sites/default/files/strategy_for_american_innovation_october_2015.pdf (accessed July 28, 2020).
- The White House. 2015. Fact Sheet: President Obama’s Precision Medicine Initiative. Available at: https://www.whitehouse.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicineinitiative
(accessed June 14, 2016). - Yin, W., J.R. Penrod, R. Maclean, D.N. Lakdawalla, and T. Philipson. 2012. Value of survival gains in chronic myeloid leukemia. American Journal of Managed Care 18(11 Suppl):S257-S264. Available at: https://www.ajmc.com/journals/supplement/2012/a386_12nov_oncology/a386_12nov_onclogy_yin_s257to64 (accessed July 28, 2020).