Quality, Safety, and Standards Organizations COVID-19 Impact Assessment: Lessons Learned and Compelling Needs
 The National Academies are responding to the COVID-19 pandemic.
The National Academies are responding to the COVID-19 pandemic.
Visit our resource center >>
Introduction
The stark reality of the COVID-19 pandemic challenged the very systems established to ensure the nation’s safety and quality. The pandemic resurfaced long-endemic challenges within the health care quality and standards ecosystem and identified novel challenges that cannot be ignored. This paper examines:
- the health care quality and standards ecosystem pre-pandemic;
- vulnerabilities in quality and standards organizations exposed by COVID-19;
- quality and standards organizations’ responses during the pandemic;
- opportunities to improve the national response to improving quality and safety overall with a particular focus on readiness in the case of disasters, pandemics, or other national emergencies;
- sector-wide policy, regulatory, and legal changes that might be transformative; and
- priorities moving forward.
The quality and safety focus of the American health care system has a long history, dating back to the 19th and early 20th centuries. Quality of care is generally understood as providing the right care for the right person at the right time—every time. Several seminal laws and publications over the last 60 years have shaped the current quality landscape in profound ways and have been cataloged in numerous publications [1,2,3,4]. These publications generally point to several landmark occurrences that have shaped the evolution of quality measurement, improvement activities, and safety standards over the years. The evolution has centered around four main levers (see Figure 1):
- survey, certification, and accreditation of facilities, laboratories, health plans, and providers;
- quality measurement, incentives, and payment reforms;
- public ratings of providers and facilities; and
- quality improvement learning and action networks.
Survey, Certification, and Accreditation
The role of government in establishing quality and safety standards for the health care system became prominent with the implementation of the 1965 Medicare legislation, in which Congress explicitly tied payment to the achievement of minimum health and safety standards, commonly known as conditions of participation (CoPs). Initially, the CoPs focused on medical staff qualifications, nursing services, and utilization review, but have since evolved to include minimum standards for various other important safety issues, including infection control, emergency preparedness, quality assurance, performance improvement, and medication management. States also established health and safety requirements for facility licensure. State standards for health and safety must, at a minimum, align with the CoPs but may go above and beyond the federal requirements.
While the state government and federal surveyors perform inspections for many facilities (primarily nursing homes), the Centers for Medicare and Medicaid Services (CMS) has approved several national accreditation organizations (AOs) to perform survey functions [5]. One of the largest AOs is the Joint Commission. Founded in 1951, the “Joint Commission accreditation can be earned by many types of health care organizations, including hospitals, doctor’s [sic] offices, nursing homes, office-based surgery centers, behavioral health treatment facilities, and providers of home care services” [65].
In 1990, the National Committee for Quality Assurance (NCQA) was established as a nonprofit organization that accredits quality programs for health plans, physicians, and other providers. NCQA developed the first set of standards for health plan quality using a set of evidence-based requirements and measurements.
The Evolution of Quality Measurement, Incentives, and Payment Reform
Despite the establishment of standards to ensure high-quality care throughout the 1980s and 1990s, numerous academic research studies demonstrated the substantial burden and threat that poor-quality care (often described as overuse, underuse, and misuse) continued to have on public health [9]. The culmination of this body of research was the publication of the Institute of Medicine (IOM), now National Academy of Medicine, report “To Err is Human” in 1999, followed several months later by the IOM report “Crossing the Quality Chasm” and the subsequent publication by Elizabeth McGlynn in 2003, demonstrating that most Americans receive less than 60% of recommended care [6,7,8].
The response to these disturbing findings was significant, beginning with an accelerated effort to measure the quality of care in the United States, to make those measurement results transparent through public reporting, and ultimately to tie payment to performance on quality and cost. Quality measurement has an established academic pedigree beginning in the 1960s with the structure/process/outcome Donabedian framework [10]. In recent years, the focus has shifted to measuring health outcomes, most notably including patient-reported outcomes [11]. This focus also includes cost, and patient experience to focus improvement efforts and facilitate comparisons between hospitals, health plans, and other organizations.
Another important dimension of quality measurement involves recognizing the challenges that communities of color, as well as people with low incomes, low levels of education, and other social drivers of health, experience in achieving optimal health and health care [13]. An integral part of delivering high-quality health care includes gaining an understanding of the social determinants of health (SDOH) of patients and communities in their respective contexts. SDOH are defined by the World Health Organization (WHO) as the “conditions in which people are born, grow, live, work, and age” [12]. However, while measuring quality has increased in importance across health systems, efforts to implement measures and metrics reducing health inequities have been lacking [43].
Increasingly, payers are tying performance on quality measures to payment, which continues to evolve in the effectiveness of connecting payment to performance to improve quality under the fee-for-service system. The move toward alternative payment models (i.e., payment approaches that provide added payment incentives for high quality, cost-efficient care by public and private payers) has had more success but has been slower to spread in many parts of the country [14].
Despite its potential value, performance measurement currently faces multiple challenges, including a proliferation of measures leading to confusion and inefficiency in reporting and collecting data, the burden associated with capturing and reporting data, and the lack of appropriate data to calculate important metrics. Lack of alignment between public and private payer measures often places providers and clinicians in the position of reporting on multiple slightly different measures that may not provide value to clinical care delivery [15]. In fact, in 2017, CMS began addressing these issues through its Meaningful Measures Framework that includes criteria to reduce measures that have achieved a high rate of performance across the majority of providers, are no longer supported by evidence or are supported by a better measure available, show the cost outweighs the benefit, or other such criteria [54].
Broad adoption of electronic health records (EHRs) by hospitals and clinician practices has been an important accelerator of quality measurement and assessment. However, the authors of the paper would agree that the pace of improvement has been incremental rather than transformational. Important gaps in the adoption of EHRs persist, and experience with the use of electronic quality measures has been challenging. However, with the maturation of digital standards and development of platforms for the exchange of electronic data, the use of digital measures holds promise for the future of reducing the burden and improving alignment and value in clinical care.
Public Ratings of Providers and Facilities
Public reporting of quality measures and standards grew out of a need for transparency in the health care system as questions began to rise from consumers about care practices. NCQA was one of the first to develop “report cards” for health plans starting in the early 1990s to address appropriate delivery of needed care. In 2003, the Consumers Union Safety Campaign developed a model law and promoted state laws to publicly publish hospital infection rates and raise public awareness about the problem. This campaign also led to changes in the Medicare program to require public reporting of hospital-acquired infections.
Today, consumer-facing public reports provide demographic and performance information about a clinician or health care facility to help people make choices. An additional, albeit indirect, impact of these reports is an increased focus on improvement by providers [55]. There is also an ongoing effort to ensure that report cards are meaningful and presented in a transparent, user-friendly manner with the intent of helping patients and families make decisions and increasing their somewhat low rates of consumer use [3].
Public ratings of the quality and safety of health plans and providers have exploded in the past several years through the development of star ratings on the CMS Compare sites (recently consolidated into one site known as Care Compare), U.S. News and World Report ratings, and the Leapfrog Safety ratings, to name a few. Notably, these systems do not consistently agree with one another [40]. Multiple studies have found that many people do not routinely consult these reports, but the percentage of people surveyed who do find quality performance reports useful has grown significantly over the past 20 years. An ongoing challenge is clarifying which areas and circumstances (e.g., need to choose a health plan) consumers find most compelling and how to present relevant information most effectively.
Quality Improvement Learning and Action Networks
While regulations stemming from the original Medicare law and subsequent statutes established a minimum floor of quality and safety for health care facilities, there was a recognition of the progress needed to drive improvements in care [52]. In 1972, Congress established the Professional Standards Review Organizations, which evolved into the modern-day Quality Improvement Organizations (QIOs) to improve the effectiveness, efficiency, economy, and quality of services delivered to Medicare beneficiaries. Under the direction of CMS, the QIO Program is one of the largest federal programs dedicated to improving health quality for Medicare beneficiaries. The program has evolved from a reliance on inspection and chart review to using quality improvement science for learning and action [3].
The Institute for Healthcare Improvement (IHI) has increasingly influenced the spread of best practices and the establishment of a culture of safety within health care organizations [16]. The Agency for Healthcare Research and Quality (AHRQ) was established in the 1990s to study and contribute to health care quality improvements [56]. Before being explicitly required by the Affordable Care Act, multiple federal components, including Federally Qualified Community Health Centers, the Veterans Health Administration, Defense Health Agency, Indian Health Services, and CMS have collaborated to improve quality and safety [46,47,51]. Both public and private sector efforts have resulted in selected sustained improvements in quality and safety and new approaches to improvement, such as substantial reductions in central line-associated bloodstream infections and patients’ experiences of care [48].
Vulnerabilities in Quality and Standards Organizations Exposed by COVID-19
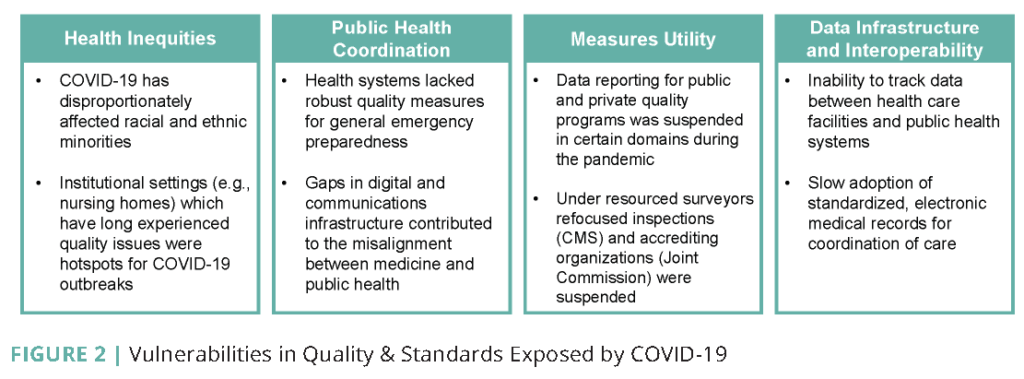
The COVID-19 pandemic brought to light the importance of the various levers for achieving quality and safety and the vulnerabilities that exist in the current system. Following the declaration of the COVID-19 pandemic by the World Health Organization, the response from the public and private health care sectors was significant. Health care professionals, government agencies, and public health officials quickly began to implement traditional means of ensuring quality and safety only to realize that standard quality metrics and emergency preparedness requirements would not be sufficient for the escalating needs of this global pandemic. Existing vulnerabilities in the quality measurement, assurance, and improvement system were magnified, calling for attention to health and health care inequities, lack of coordination between public health and health care delivery quality and safety metrics, effectiveness, and inadequate data infrastructure and interoperability (see Figure 2).
Health and Health Care Inequities
COVID-19 has further revealed widespread health inequities that are impossible to ignore. Rates of COVID-19 infection have been as much as two to three times higher in Black, Latinx, Asian-American and Pacific Islander, and Indigenous communities compared to their White peers [17, 18]. The CMS data snapshot from January 1, 2020, through August 15, 2020, reveals that Black beneficiaries have more than twice as many cases per 100,000 than White beneficiaries with Black beneficiaries recorded at 2,799 per 100,000 compared to 1,272 per 100,000 for White beneficiaries. Indigenous communities, listed by the study as American Indians/Alaskan Natives, had a rate of 2,152 per 100,000 with Hispanic cases at 2,627 per 100,000 [34].
The reasons for these disparities are multifactorial and long-standing. Before entering the health care system, communities of color usually experience poorer levels of health. For example, Black, Latinx, and Indigenous individuals often experience a higher degree of comorbidities compared with White peers, increasing their vulnerability to serious disease. These disparities are caused by social, political, and economic inequality that is underpinned by decades of structural racism and neglect [21].
Communities of color and communities that have been made to be vulnerable experienced higher incidence of infection with COVID-19 than their White peers. The most proximal cause was the exposures of these communities to sources of COVID-19 infections because of their concentration in person-facing essential jobs in the service sector, including workers in public transit, transportation, logistics, food, beverage, janitorial services, and childcare and social services. These jobs, which often cannot be performed from home and require interaction with people, also often do not provide paid sick leave or family leave, adequate protective equipment, or comprehensive health insurance and health care services. Importantly, while, as described above, communities of color and communities that have been made to be vulnerable may have needed to interface with health care services more than their White counterparts during the COVID-19 pandemic, these groups have been and continue to be harmed or neglected by the health care system at higher rates than their White counterparts, leading to a lack of trust in health care institutions and professionals.
Also concerning is the fact that communities of color and communities that have been made to be vulnerable often experience higher barriers to accessing quality care. Initial research has highlighted the increase in mortality from COVID-19 because of these access gaps—clinical disparities in the same studies reduced once individuals from these communities received the necessary treatment for the disease [39]. The impact on in-hospital mortality among those infected has been mixed, with population-based studies demonstrating higher death rates for persons of color, while studies from the Veterans Health Administration and the Ochsner Clinic in Louisiana found no significant mortality differences [19]. Notably, these findings do not capture potential long-term morbidity issues, such as cardiac function and cognitive problems.
For decades, the calls to address the inequality in health and access to care for these populations have gone largely unanswered. The IOM called for action to address health care disparities in its 2003 report, Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care [32]. The report notes that Black, Latinx, and Indigenous populations tend to receive a lower quality of health care than White populations, even when controlling for access-related factors, such as patients’ insurance status and income [32]. Key barriers to obtaining high-quality treatment include racial biases and stereotyping, as well as language and geography barriers [32]. By law, AHRQ has reported to Congress annually on health care disparities since 2003, with each successive report finding no or only marginal improvements.
Ten years after the publication of Unequal Treatment, the Centers for Disease Control and Prevention (CDC) published the 2013 Health Disparities and Inequalities Report, which examined “disparities in deaths and illness, use of health care, behavioral risk factors for disease, environmental hazards, and social determinants of health at the national level” [33]. Due in part to the endurance of these problems despite public health research efforts, terms such as cultural familiarity and behavioral risk factors for disease are falling out of favor and being replaced with holistic concepts about root causes, such as racial bias, structural racism, and implicit bias that are drivers of increased morbidity and mortality for communities of color at the national level.
Public Health and Health Care Sector Coordination and Measures Effectiveness
The pandemic demonstrated the stark divide between our public health and clinical care systems. For decades, public health has been underfunded at all levels of the government, which hampered U.S. pandemic preparedness and response. In addition, the quality and safety focus areas for public health and clinical care have been poorly aligned, with health systems focused more on specific clinical areas such as treatment for acute cardiac conditions and avoidance of localized nosocomial infections; public health systems are traditionally more focused on communicable disease control and prevention of chronic disease and injuries. There was no existing data infrastructure across these systems that included key variables and metrics around readiness to inform preparedness and the response capacity of the health care system. As a result, most health systems did not have data or systems for collecting and sharing information about personal protective equipment (PPE), essential staffing, or ventilator shortages at the start of the crisis. The locus of control between the federal, state, and local governments was not always clear, with emergency response responsibilities resting in multiple federal government agencies and varying forms of state-local governance structures for public health, which can generally be described as centralized, decentralized (or home rule), mixed, and shared state and local governance. Each model presents its unique coordination challenges. The state-local mixed models are described in more detail in Public Health COVID-19 Impact Assessment: Lessons Learned and Compelling Needs [37].
Within the health care sector, multiple organizations and systems compete for business in a market-based system, with little guidance, much less metrics, for cooperation and collaboration during a public health emergency [44].
The broad quality frameworks for payers and providers have created a large infrastructure and set of resources that focus on tackling dissimilar requirements and metrics across markets and providers, supporting the disparate data and reporting systems. The resources devoted to this enterprise generate revenue and enable provider incentives—but this “teaching to the test” does not contribute enough to the health of individuals, and activities such as support for SDoH are not always rewarded.
The complex and layered measurement system was largely built through federal and state legislation. Measurement currently serves multiple agendas. The wide-ranging focus and cumbersome data capture and reporting system leave the health sector unable to tackle novel or emerging specific issues, particularly crises such as COVID-19, which required such comprehensive cross-sector collaboration. Without an overarching strategy or direction, the current system is one in which no outcomes are prioritized—there is no ability to create consistent directional change for the health of a population.
Data Infrastructure and Interoperability
While the U.S. maintained a national stockpile of PPE and ventilators, the lack of a digital and communications infrastructure for early detection and preparation for the COVID-19 pandemic, along with limited visibility of the Strategic National Stockpile, limited the ability to equip clinical settings and the public adequately. Crucial data and information did not flow between public health agencies or the medical care system and standards organizations. Additionally, a lack of investment in systems to support digital data capture in post-acute care settings widened the quality information gap. Lack of testing contributed greatly to the rapid spread of COVID-19. Still, even when testing was available, the spread occurred much earlier and more widely than could be known because of the lack of an infrastructure to surface and share such data.
Although everyone performing a COVID-19 test captured test results in digital format, the systems and rules governing the flow and aggregation of those data were not sufficient for the task of tracking the pandemic in real time. Instead, new platforms emerged using person-to-person outreach and mining data from dozens of sources manually to produce trusted tracking information.
Long-standing and oft-lamented shortfalls such as insufficient interoperability, slow creation and adoption of data standards, inadequate adoption of EHRs across the health care system, and a need to focus more precisely and consistently on the most urgent quality issues now stand out even more starkly than before the pandemic. One paper noted that among the weaknesses highlighted by the pandemic is the inability of the U.S. to develop clinical guidelines, related decision supports, and quality measures quickly [23]. EHRs, registries, health information exchanges, artificial intelligence, and other tools are insufficiently leveraged to gather syndromic surveillance data and assess trends in near-real time. These same weaknesses have contributed to the inability to develop and deploy digital quality measures across care settings pre-pandemic, which could address the shortcomings of traditional quality measures such as the lack of inclusion and exchange of clinical information to drive substantive quality and safety improvements. These shortcomings of the quality infrastructure left our nation without ongoing insight into and accountability for safety and quality during a crisis.
Quality, Safety, and Standards Organizations Sector Response during the COVID-19 Pandemic
The quality, safety, and standards community was called upon to respond quickly to mitigate and control the COVID-19 pandemic. CMS and other payers suspended data collection for some quality measures to help ensure that providers were singularly focused on patient care. Underresourced federal and state survey and oversight activities shifted primarily to a focus on infection control. AOs that typically conduct an in-person assessment either suspended surveys or shifted to a virtual mode for some cases. Federal and state surveyors continued onsite surveys but combined them with virtual surveys for those that were more focused on critical areas such as infection control and abuse and neglect. Payers, including CMS, liberalized telemedicine policies to ensure access to care while minimizing the exposure risk of both clinicians and patients. The impact of these policy changes on the quality and safety of patient care is unknown and will need to be studied, including its impact on people without reliable or sufficient access to information and communications technologies.
To increase the ability to care for patients with COVID-19, hospitals canceled elective surgeries and, where possible, expanded the physical capacity to care for the expected influx of patients and to separate physically those infected with COVID-19 from other patients. In addition, many hospitals and other facilities enacted policies intended to safeguard the health and safety of health care workers, patients, and their family members, such as limitation of visitors and other personnel in direct patient care areas and universal symptom screening of all entering a facility.
The following describes the sector’s response using the following levers:
- survey, certification, and accreditation of facilities, laboratories, health plans, and providers;
- quality measurement, incentives, and payment reforms; and
- quality improvement learning and action networks.
Survey, Certification, and Accreditation
Hospitals and other providers are required to meet minimum standards for emergency preparedness as part of the Medicare CoPs. The CoPs currently require that health care providers collaborate with appropriate local authorities as part of their emergency preparedness policies. However, the pandemic has demonstrated that this collaboration is often ineffective or absent, again likely due to a lack of data sharing and communications infrastructure. There is an opportunity to address these deficiencies through a more intentional alignment of safety priorities and incentives between public health and clinical health care systems.
Accrediting bodies also had to shift their focus and operations rapidly in response to COVID-19. One of the largest AOs, the Joint Commission, suspended routine in-person surveys for health care organizations to enable health systems to prepare and implement rapid COVID-19 response efforts. For organizations with expiring accreditation status, the Joint Commission extended accreditation due dates to prevent the disruption of Medicare payment mechanisms [57]. Limited accreditation surveys resumed in June 2020, with virtual surveys being tested in several sites [59]. The resultant impacts of virtual surveys on the public’s health and safety remain unknown and should be studied. Attention should also be paid to the effectiveness of accreditation requirements to determine which requirements should be retired in favor of standards that reflect health care system readiness for future pandemics and are more likely to support quality and safety.
In March 2020, CMS also suspended nonemergency inspections, allowing inspectors to focus on COVID-19 and abuse [59]. The impact of these suspensions and narrowing of focus will be evaluated over time for continuous improvement. Still, future analyses will likely reveal which quality areas should be prioritized and which may be less important to the overall health and safety of patients and residents. This transformation could be an important opportunity to remove requirements that are of little to no value.
CMS placed particular focus on nursing home surveys because they are both a care setting and a full-time home for their residents. Over 100,000 deaths from COVID-19 occurred among nursing home patients, thought to be related to the challenges of controlling infections in a congregate setting, physical infrastructure challenges, and health-related challenges of frail older people. Prior to COVID-19, fewer than 4,000 of the nation’s 15,400 Medicare-certified nursing homes voluntarily reported health care-associated infections (HAIs) to the CDC’s National Health Safety Network (NHSN), which provides health care facilities with a system to track infections and prevention measures.
The CDC rapidly developed a new COVID-19 module for reporting data that subsequently became required for reporting through an Interim Final Rule with Comment published on May 8, 2020. Under this rule, noncompliance on reporting standards could result in the imposition of civil money penalties [45]. Within weeks, at least 95% of nursing homes began to report data in the four pathways within NHSN’s Long-term Care Facility Component, providing valuable information on “resident impact, facility capacity, staff and personnel, supplies and PPE, and ventilator capacity and supplies” [50].
The CDC data are critical to guiding nursing home-focused surveys, training, resource allocations, and quality improvement activities and interventions. As of November 21, 2020, states had completed 42,267 surveys. While most surveys were conducted in-person, virtual surveys were also conducted to provide rapid assistance. Survey findings often pointed to a breakdown in basic infection control processes such as proper hand hygiene, doffing and donning PPE, social distancing, staff screening, and precautions [49]. The findings point out that it is not sufficient to just have regulations in place. Training, technical assistance, oversight, and enforcement must also be in place to ensure adherence to quality and safety standards. While reporting of HAIs through the NHSN is not currently required by CMS, there is now an opportunity to take regulatory action to implement such a requirement. While this may add a burden to providers, a review of the impact of the suspension of other requirements may demonstrate less impactful requirements that could potentially be retired.
The authors observed that laboratory entities were somewhat burdened by testing regulations for the CMS. Laboratory testing on humans, except for research, is governed by the CMS through the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and is estimated to include around 260,000 CLIA-regulated laboratory entities [60]. During the pandemic, the CMS established new reporting requirements for hospital and critical access hospitals CoPs to enable the collection of COVID-19 incidence and impact data. The requirements compelled all CLIA-regulated laboratories to report COVID-19 testing results directly to the HHS Secretary [61].
While the reporting is necessary to determine the prevalence of COVID-19 in the community, these reporting requirements attracted criticism for their substantial impact on CLIA-regulated laboratory entities. Providers noted that the reporting of COVID-19 laboratory data was initially burdensome and not well coordinated, resulting in multiple reporting platforms and potentially duplicate reporting between federal, state, and local requirements. In addition, providers who used point-of-care antigen testing were required to obtain a Certificate of Waiver and follow the CLIA requirements, established by Congress, for which some providers had no prior experience.
Quality Measurement, Incentives, and Payment Reform
The pandemic created unique demands on the health care and public health sector, raising questions about the current system’s long-term sustainability and its ability to coordinate activities across sectors. Quick action halted quality efforts that were not specifically necessary during the pandemic so that providers could focus on caring for patients. Health care quality data reporting was mostly suspended, first by CMS but followed rapidly by private payers and states. During COVID-19, payers recognized that quality measure data collection and reporting for services furnished might not reflect the true performance level on measures such as cost, readmissions, and patient experience. One paper suggested that suspension was the only practical response because so much of current quality reporting is still dependent on manual processes considered overly burdensome [22].
However, some suspensions warranted reversal as the pandemic persisted. One such example was the suspension of the Payroll-Based Journal system staffing data for nursing homes. Although initially suspended, the waiver of nursing homes’ requirement to submit staffing data through the Payroll-Based Journal system was rescinded in August 2020, and nursing homes were required to begin to submit data by August 14, 2020. In this case, the need for staffing data for nursing homes because of staffing shortages outweighed the potential burden during a public health emergency.
While the ease, timeliness, effectiveness, and relevance of quality measures remain a critical issue for analysis and alignment, the importance of measurement for quality improvement and system-wide action should not be underestimated. While there were no direct measures associated with pandemic performance, quality measurements can indicate poor systemic performance during a pandemic. For example, CMS publishes health inspection and quality ratings based on their Five-Star Quality Rating System for all CMS-certified nursing homes. A study conducted by the CDC during March 2020 through June 2020 of 123 outbreaks in West Virginia nursing homes concluded that star ratings may be correlated with COVID-19 outbreak risk; facilities with 2- to 3-star and 4- to 5-star ratings were less likely to experience a COVID-19 outbreak by 87% and 94% respectively than 1-star facilities. The CDC concluded that because star ratings are a composite measurement tool, there is an opportunity to learn which health standards measures had the most significant impact. In addition, this small study suggested further efforts to improve the current systemic problems of inequitable access to quality care and good health along with the various social drivers of health [24].
While some measures were suspended, other quality measures and incentives were developed to encourage clinician participation in deploying novel treatments and therapeutics. CMS leveraged its existing value-based care initiatives and payment and reimbursement mechanisms to promote COVID-19 vaccination as part of their ongoing value-based care initiatives for Medicare Exchange and Advantage plans [62]. CMS also partnered with the CDC to incorporate patient and personnel vaccination as part of quality measures in nursing homes and dialysis facilities [62]. To encourage a culture of quality care and outcomes, the CMS Merit-based Incentive Payment System Program also began offering credit for clinicians participating in COVID-19 clinical trials and registries [64]. In March 2021, Medicare began paying approximately $40 per required dose of COVID-19 vaccines [63]. While the overall impact of these efforts remains to be seen and should be systematically assessed, the registries have presented a compelling and innovative mechanism to obtain new information and datasets related to the COVID-19 pandemic.
Quality Improvement Learning and Action Networks
Quality improvement networks were activated immediately to provide training and support to the health care system, with particular attention to nursing homes. CMS directed the Quality Innovation Network-QIO (QIN-QIO) and the CMS Quality Improvement Contractors, to focus their technical assistance on providing nursing homes with onsite or virtual training in areas of identified concern, particularly in COVID-19 outbreak hotspots [53]. CMS also worked with the CDC to implement a national training program for nursing home staff and management.
States also provided intensive assistance to nursing homes through the CDC-supported state-based HAI prevention programs. State coordinators worked closely with public health partners to assist with data analysis, infection prevention and control processes, and real-time support.
In September 2020, AHRQ partnered with the University of New Mexico’s ECHO Institute in Albuquerque and IHI to establish the National Nursing Home COVID-19 Action Network [25]. Through this collaboration, the network delivers a 16-week training program to increase the adoption and implementation of evidence-based prevention, response, and treatment practices to protect nursing home residents and staff and reduce social isolation [25].
These examples of using quality improvement learning and action networks could potentially facilitate the dissemination of critical prevention and response strategies, and treatment guidelines, while including other considerations unique to nursing home residents and COVID-19, such as mental health, HAIs, and the increased likelihood of severe disease.
Opportunities for Improvements in Quality, Safety, and Standards Sector
The pandemic revealed a profound lack of infrastructure for evaluating the public health system and significant failings of the current quality measurement infrastructure for the health care delivery system. These systems need to get stronger and smarter—time is of the essence in distilling a full understanding of opportunities for improvement and to implement structural change.
The following are a set of urgent actions that can reinforce and improve the quality, safety, and standards sector, focused on:
- ensuring strategy and infrastructure preparedness;
- digitizing and sharing critical information across sectors;
- improving population health measures;
- streamlining metrics; and
- addressing inequities that can be taken to transform readiness, bolster the public health infrastructure, and improve health outcomes.
Ensuring Strategy and Infrastructure Preparedness
To address the critical vulnerabilities in America’s public health preparedness, we can no longer implement infectious disease preparedness by applying superficial lessons learned from past infectious disease threats and ecological disasters toward future and novel threats. Instead, we should revisit the consensus definition of preparedness at the national, state, and local levels, with attention to planning and execution and robust health surveillance and vulnerability detection.
Health systems, leaders, and professionals must expand our understanding of preparedness to include readiness for and responsiveness to emergent and sustained threats. Preparedness must begin to signify the ability to rapidly develop and deploy a dynamic and responsive action plan to meet existing and emerging challenges. These challenges are likely to include simultaneous threats to public health in the form of climate change, regional and ecological reservoirs of known and novel disease, national and regional outbreaks and epidemics, global pandemics, mass refugee migration, and cyberthreats. In many instances, such a strategy should enable local flexibility to tailor responses matched with local threats, needs, and assets while accounting for the risk of emerging infectious diseases globally.
A new preparedness measurement strategy should include structural measures for enhanced preparedness, process, and outcome measures to support emergency responses. Derived from a global preparedness plan or strategy, there should be a set of implemented and functional structures to support the strategic goals. There also should be process measures designed to assess the adequacy of response to disasters and other public health threats.
Finally, the adequacy of the preparedness and response to an event should also be assessed according to individual and population outcomes. These measures might include both short-term metrics to assess interventions with rapid results over the days and weeks immediately following an emergency. Metrics could capture long-term measures such as six-month hospitalization and mortality following disasters or changes in health indicators for those developing chronic diseases after an emergency. To drive innovation, the preparedness measurement strategy could potentially capture the emerging capacities of virtual care and telemedicine at scale while accounting for the varying access to sufficient and reliable access to information and communications technologies.
Establishing a robust measurement strategy enabled by a new and robust data infrastructure will be critical if we are to transform our system from one that struggles through disaster, lamenting gaps and inadequacies that we only come to fully appreciate after the fact, to one that anticipates emerging threats, reacts quickly, prepares adequately, and successfully mitigates the impact on the health and well-being of our citizenry.
Digitizing and Sharing Critical Information across Sectors
Common to clinical care and public health and preparedness systems is the need for timely access to accurate digital information and efforts to increase access to this information. This access, if expanded, provides greater investment in robust and interoperable health IT infrastructure and a system of data integration delivering the right information to the right place at the right time and from every setting where health care is provided. Data would be transmitted across local, state, and regional public health departments, schools, and every outpatient health delivery entity, as well as short- and long-term institutional living facilities.
Further, this must be done in a way that maintains privacy and protects the overall system from the constant threats of cyberattacks. Reaching this goal requires continued adherence to current rules and an acceleration of additional interoperability standards and rules across the sector and public-private collaborations among data aggregators and data users to create a system and process for safe, secure data access. Transformation in interoperability and investments in data sharing infrastructure should aim to create a nimbler system to allow development, collection, calculation, and analysis of new metrics in real time to respond to emerging threats and directly apply to the crises at hand. Achieving this aim will require a sustained focus on removing barriers to a cross-sectoral approach (acute care, outpatient, post-acute, and public health) that emphasizes harmonization of data and rapidly disseminated interpretation of findings through investment and expansion of applied informatics throughout the system. Innovations and investments in data science, informatics, governance models, and cross-sectoral collaboration will enable the best epidemiologists and scientists globally to innovate new methods of distilling critical information from data to support public health.
In addition, EHRs, registries, health information exchanges, artificial intelligence and other tools must be better leveraged to gather syndromic surveillance data and assess trends and treatment efficacy in near real time. The ecosystem must also support digital quality measures to address shortcomings with traditional quality measures in all care settings.
Improving Population Health Measures
The time has also come to create and implement local, state, and regional metrics for population health status and assessment of vulnerabilities using sensible geographic demarcations such as census block groups and shared accountability among providers serving those areas. Investments must be made in the public health entities (Federally Qualified Health Centers, local health departments, correctional health services, and so forth) serving highly vulnerable areas followed by direct financial incentives and additional investments for improved performance and decreased population vulnerability over time, similar to the model for implementing EHRs. Along with a new system of metrics that increases the use of digital measures, there must be an expansion of payment incentives directed at the health care delivery system. This expansion should focus on mitigating population health vulnerabilities caused by the social and environmental determinants of health equaling or exceeding current financial incentives of fee-for-service payment models. Federal, state, and private payers must continue to modernize the health care delivery quality measurement system that, in addition to fully digitizing quality measures, applies novel technological solutions that eliminate the burden of data sharing and reporting in the public health sector. To enhance the adoption of these measures, efforts could potentially enhance population health measures for increased transparency and accessibility. Ongoing public reporting of population-level measures may promote public awareness while building support for transformations in care delivery systems.
Streamlining Metrics
The arduous process for data capture, implementation of novel measures, and removal of measures that are no longer useful must be addressed. Similarly, the multiple agendas the measurement system is serving as well as the lack of prioritization of outcomes should be further studied. It is critical to reimagine a system that supports the innovation necessary to drive system transformation, improve outcomes, and reduce cost, particularly for underserved populations and Medicaid. We should also seize on the learning from COVID-19 by evaluating current measures post-pandemic to determine if there was any relationship or predictive value to the measures and the success of nursing homes, hospitals, or other care settings to manage patients with COVID-19.
Addressing Equity
Finally, to address the inequities laid bare by the pandemic, there must be new metrics and incentives for achieving equity in health care delivery [26,27,28]. It will be important to act on the urgency created by this pandemic to develop new access to care metrics and to use well-functioning metrics and measures to accompany population and provider quality measures. De facto segregation in care, derived from structural racism in housing and lack of access to adequate care, is a substantial driver of racial and ethnic health care disparities. In addition, metrics and measures of health care workforce diversity and integration across the workforce pay scale are needed. Recent studies have demonstrated that communities of color and communities that have been made to be vulnerable experience better outcomes when receiving care from clinicians with shared identities [29].
Additional innovations should include patient-reported outcome measures focused on the experiences of discrimination in health care delivery, payment models incentivizing providers to implement innovations anticipating and addressing health care disparities, and a requirement for anti-racism training as part of provider training, and accreditation, board certification, and continuing education for individual and institutional health care providers. Inequities in care delivery can be addressed and eliminated in health care delivery with investment and commitment to make these changes.
To ensure a commitment to constantly improving health systems, research could be conducted on the effectiveness of specific training programs to ensure that efforts to address health inequities and implicit biases are informed by evidence and consistent stakeholder education. Using this evidence, a proof of concept for how health equity could be incorporated into the star ratings for Medicare Advantage Plans to offer a sound, empirically tested starting point [30]. Beyond using existing measures and indices, health equity in care research could also investigate the possibility of using new measures that reflect timely access to care, equity, and racism to inform and enable systems transformation.
Finally, health equity should be intentionally incorporated into the design of Alternative Payment Models by CMS and private payers. The CMS Innovation Center’s Accountable Health Communities model is a good start [38]. This and other models could be strengthened by an explicit goal of improving health equity, broadening the definition of the SDoH to include structural drivers of health inequities (e.g., racism, housing policies) and use a measurement framework for health equity [38,39].
Sector-Wide Policy, Regulatory, and Legal Changes That Might Be Transformative
COVID-19 was a test of whether the U.S. health care system can pivot to measure, motivate, and optimize the most important and relevant health outcomes, and the quality, safety, and standards sector did not pass the test.
Moving forward, policy changes at federal, state, and local levels in CoP reform, payment reform, and equitable distribution of resources to achieve better outcomes for underserved populations will be crucial for the transformation of the quality, safety, and standards organizations sector.
CoP Reform
While the implementation of standards for emergency preparedness for all Medicare-certified providers in 2016 was an important development, the pandemic requires policy makers to quickly understand and address gaps and potential enhancements to the current regulatory landscape. Institutionalized populations perhaps fared the worst. Settings such as nursing homes, long-term care facilities, and psychiatric care centers became environments of disproportionate spread because of a variety of factors that must be evaluated to inform ongoing enhancements in federal and state oversight of health and safety standards—for nursing homes in particular.
The current regulations were borne of a series of devastating hurricanes (Katrina, Sandy) and expanded due to an all-hazards approach because of Ebola and H1N1. However, in the context of an ongoing, chronic disaster such as COVID-19, current regulations need ongoing review to consider prolonged stresses on the health care system and on health care workers. CMS provided over one hundred flexibilities through Section 1135 waivers of certain CoP requirements in an effort to expand available sites of care, address workforce capacity and scope of practice barriers, and help health care providers contain the spread of COVID-19. Ongoing efforts are necessary to determine if any of the flexibilities should be made permanent and additional efforts necessary to continually strengthen requirements around infection control and prevention, worker safety, repeated surges of patients, and especially coordination and collaboration across health care providers within communities and with local organizations. Similarly, states should review their licensure requirements for long-term care facilities and other health care institutions to identify enhancements that go above and beyond federal requirements depending on their local experiences and needs.
Payment Reform
The Health Care Payment Learning & Action Network, a collaborative network of public and private stakeholders, note that the health care system must reform payment structures, mechanisms, and incentivizes to encourage value, quality, and outcomes. These components can produce a system, which spends strategically to deliver better care for a healthier population. Transforming the health care system requires system-wide and multisectoral collaboration and partnerships to reimagine care organization and delivery [31].
The fee-for-service payment model encourages waste, discourages innovation, and makes it difficult for practitioners and systems to know which patients they are accountable for. Systems and practitioners in a capitated environment benefit from successful efforts to make care delivery more efficient. They know who their patients are, understand the budget they must operate within, and have clear incentives to coordinate care and drive toward the best outcomes.
The last several years have seen numerous experiments in alternative payment models implemented by public and private payers with varying degrees of success—some quite successful—defined as lowering costs and improving quality. Given the inequities and quality shortfalls that the pandemic has exposed, now is the time to double down on payment reform and accelerate the move toward systems with the capabilities of capitated systems. Incentives for better coordinated care, moving care into the home, seamless sharing of data and information across providers, and reduction of waste are essential to the sustainability of our health care system and to optimize our ability to keep people healthy during a pandemic or other disaster. A closer look at the need for health care reform can be found in Health Care Payers COVID-19 Impact Assessment: Lessons Learned and Compelling Needs [37].
Equitable Distribution of Resources to Achieve Better Outcomes for Underserved Populations
Based on the long-standing records of health care disparities in the U.S., it should be no surprise that the impact of COVID-19 disproportionately affected Black, Latinx, and Indigenous populations. Although many causes of health disparities often originate outside the health care system because of structural racism and discrimination, it is the responsibility of health systems and care practitioners to address the specific needs of:
- Black/Latinx populations;
- Native and tribal communities;
- individuals residing in congregate or institutional settings (e.g., prisons, nursing homes, mental health facilities) [20]; and
- people in rural health settings.
Despite the formidable challenge, the U.S. must establish quality measures and standards to address pre-existing issues combined with the devastating effects of a pandemic. First, there must be conscious efforts by all to focus on acknowledging and owning responsibility for disparities and a commitment to eliminating them. There is little need for additional studies to prove that disparities exist, but there is a great urgency to eliminate them. It must be acknowledged that racism is embedded in systematic ways both in the health care delivery system and in society broadly that impact adequate access. Health care organizations cannot solve these challenges without the help of public health and community organizations and must also work with community leaders who have the requisite trust with the community to develop sustainable solutions. Addressing racism and health disparities does not lend itself to a purely medical model, a list of quality measures that focuses only on the disease process or interventions that rely on an individual clinician’s actions. It will be imperative to articulate and execute a shared accountability approach that involves cohesive action from multiple actors and their interdependencies across social, economic, and political systems. This unified approach should be supported by sector-specific time-bound goals, actions, metrics, and incentives.
Learnings must be identified and documented as the system adjusts to address urgent needs rising from the pandemic. Then, when the pandemic is under control, the system should apply those learnings toward the transformation of health care and other sectors to achieve greater equity. For the immediate needs, the CDC outlines certain recommendations for addressing disparities during the COVID-19 pandemic, such as ensuring increased testing, contact tracing, isolation, and disease management in populations more likely to contract COVID-19 [44]. Ideally, data must drill down to the county and neighborhood levels to ensure the equitable distribution of testing, contact tracing, and vaccination. These targeted responses are critical and do not support the frequently held notion that improvements to the system overall will somehow reduce disparities. There must be relentless efforts to implement anti-racist policies to combat the earned mistrust of health care and public health interventions in Black, Latinx, and Indigenous communities.
There is also a demonstrable link between vaccine acceptance and the perception of fairness, experience of racism in health care, and perception of one’s own risk of being the victim of discrimination within Black communities [42]. However, health care providers have not engaged in efforts to address the problems that continue to drive inequity directly. Communication about the importance of vaccine administration and safety must come from trusted sources [35]. There must be targeted logistical efforts to achieve equity in making vaccines available and safely administered to communities of color and communities that have been made to be vulnerable, as well as ensuring appropriate and continual follow-up with these communities. These accommodations could include workplace vaccinations and incentives to help people who cannot miss work to receive vaccines during workdays. Data and metrics must be closely followed and made publicly available to provide reliable information on progress in this area or lack thereof [36].
Post-pandemic activities must include collective and earnest efforts to address inequities and health disparities that existed prior to COVID-19. These efforts include opportunities to reduce inequities including development and refinements of disparities indexes and then holding providers and health systems accountable by tying reduction of disparities to payment. In addition, the expansion of digital capture of health information affords an opportunity to capture information more fully about the exposure and impact of individual interactions with systemic racism across sectors. For example, it is possible to digitally map patients to residence in historically redlined and subsequently economically divested communities, to capture experiences of interaction with police and corrections systems as well as performance of public primary and secondary schools that children attend. Collecting these data in health care and public health sectors can provide essential information to improve prevention and health outcomes. This information could also inform efforts to develop holistic efforts targeted toward geographic areas most harmed by systemic racism.
The expansion of enrollment and use of patient portals during COVID-19 could be leveraged to capture patient-reported information about trust in providers, experiences of racism, perceived risk of discrimination, and other critical predictors of access to preventive care among disadvantaged groups. Attention to metrics in this area becomes particularly important as goals for payment move away from fee-for-service models to value-based care.
Along with indisputable gaps in the current quality, safety, and standards organizations ecosystem, the pandemic also revealed heroic responses of clinicians and frontline health care workers, as well as their leaders. In addition to providing clinical care, many contributed to journals and online platforms to share key insights and lessons learned, led efforts to locate PPE, and identified childcare options for clinicians who had to work through the most intense surges of COVID-19. Moreover, early in the pandemic, thousands of health care professionals volunteered to serve in the hardest-hit communities. More recently, many clinicians are energetically identifying and addressing misinformation propagated through social media. Priorities for moving forward must recognize the importance of professionalism generally and these contributions specifically, and anticipate the observed fatigue and burnout experienced by many providers. It will also be essential for leaders to restore trust with clinicians who did not feel supported throughout the pandemic.
Priorities Moving Forward for the Quality, Safety, and Standards Organization Sector
COVID-19 has presented an incredible opportunity to rethink the quality, safety, and standards organizations sector’s design and functioning. The health ecosystem must respond to the urgent call for equity in health care delivery and health outcomes by defining goals, developing and deploying metrics, and aligning resource distribution and payment policy. The most urgent priorities include:
- Ongoing systematic review of CoPs in consideration of requirements related to prolonged stresses on the health care system, including how various care settings can work more closely together to address issues of a surge of hospitalizations, support for home care, and coordination of discharge or transfer from one setting to another.
- Acceleration of the work begun pre-COVID-19 to strengthen and modernize the quality measurement strategy and infrastructure. Investments in and design of new models and standards for collaboration between health care and public health systems should be significantly increased. These collaborations should be fueled by and assessed with digital data to drive learning, innovation, and impact.
- Expanding and rapidly accelerating the shift away from fee-for-service models and toward alternative payment that promotes value and optimizes population wellness, resilience, and patient outcomes. Payment reforms to facilitate quality improvement should be aligned with specific strategies to reduce known disparities and promote equity. Payment reforms should be tracked with metrics that can assess performance at the individual, population, and health system levels.
- Investing in the expansion of digital data capture in public health and congregate settings, ensuring the data are fully interoperable and expanding public-private partnerships to accelerate innovation and agility in the digital information and measurement space. Finally, these changes must be driven by prepared, capable, and committed leaders from across all sectors. An improved and agile data infrastructure must be guided by policy interoperability across sectors, the commitment to shared goals, and recognition of critical interdependencies.
Building a renewable source of leaders in the public and private sectors can be enhanced by cross-disciplinary and multidisciplinary leadership training programs and experiences to foster shared learning by health care business leaders, public health leaders, and clinical leaders beginning with early stages of career development. Attention should be given to the skills that will be needed to drive significant change, including anti-racism, behavioral economics, rapid-cycle testing and learning, practice transformation, and change management, to name a few.
With robust strategic investments and partnership by the public and private sectors and a cadre of leaders committed to transformational change, the quality, safety, and standards organization sector can emerge from the COVID-19 pandemic smarter, fairer, and stronger than before.
Join the conversation!
![]() Tweet this! Quality, safety, and standards orgs leaders outline priority actions after #COVID19, including new models/standards for collaboration between health care & public health and accelerating shifting away from fee-for-service care: https://doi.org/10.31478/202107d #TransformingHealth
Tweet this! Quality, safety, and standards orgs leaders outline priority actions after #COVID19, including new models/standards for collaboration between health care & public health and accelerating shifting away from fee-for-service care: https://doi.org/10.31478/202107d #TransformingHealth
![]() Tweet this! A new #NAMPerspectives outlines vulnerabilities in quality, safety, and standards orgs exposed by #COVID19, including health inequities, public health coordination, measures utility, and data interoperability. Read more: https://doi.org/10.31478/202107d #TransformingHealth
Tweet this! A new #NAMPerspectives outlines vulnerabilities in quality, safety, and standards orgs exposed by #COVID19, including health inequities, public health coordination, measures utility, and data interoperability. Read more: https://doi.org/10.31478/202107d #TransformingHealth
![]() Tweet this! Quality, safety, and standards org leaders outline the sector’s response to #COVID19 in a new #NAMPerspectives, including suspending data collection to ensure that clinicians could focus solely on providing care. Read more: https://doi.org/10.31478/202107d #TransformingHealth
Tweet this! Quality, safety, and standards org leaders outline the sector’s response to #COVID19 in a new #NAMPerspectives, including suspending data collection to ensure that clinicians could focus solely on providing care. Read more: https://doi.org/10.31478/202107d #TransformingHealth
Download the graphics below and share them on social media!
References
- Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
- Institute of Medicine. 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press. https://doi.org/10.17226/10027.
- Moody-Williams, J. D. 2020. A Journey Towards Patient-Centered Healthcare Quality: Patients, Families and Caregivers, Voices of Transformation. Springer Nature: Switzerland.
- McGlynn, E. A. 2020. Improving the Quality of U.S. Health Care—What Will It Take? New England Journal of Medicine 383:9. https://doi.org/10.1056/NEJMp2022644.
- Centers for Medicare and Medicaid Services (CMS). n.d. Accreditation of Medicare Certified Providers & Suppliers. Available at: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Accreditation-of-Medicare-Certified-Providers-and-Suppliers (accessed January 4, 2021).
- Lohr, K. N., and S. A. Schroeder. 1990. A Strategy for Quality Assurance in Medicare. New England Journal of Medicine 322:707-712. https://doi.org/10.1056/NEJM199003083221031.
- Leape, L. L., T. A. Brennan, N. Laird, A. G. Lawthers, A. R. Localio, B. A. Barnes, L. Hebert, J. P. Newhouse, P. C. Weiler, and H. Hiatt. 1991. The Nature of Adverse Events in Hospitalized Patients—Results of the Harvard Medical Practice Study II. New England Journal of Medicine 324:377-384. https://doi.org/10.1056/NEJM199102073240605.
- McGlynn, E. A., S. M. Asch, J. Adams, J. Keesey, J. Hicks, A. DeCristofaro, and E. A. Kerr. 2003. The quality of health care delivered to adults in the United States. New England Journal of Medicine 26;348(26):2635-45. https://doi.org/10.1056/NEJMsa022615.
- Chassin, M. R., and R. W. Galvin. 1998. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA 280(11):1000-1005. https://doi.org/10.1001/jama.280.11.1000.
- Donabedian, A. 1988. The quality of care. How can it be assessed? JAMA 260(12):1743-1748. https://doi.org/10.1001/jama.260.12.1743.
- U.S. Food and Drug Administration. 2009. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims: Guidance for Industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (accessed January 4, 2021).
- World Health Organization. n.d. Social Determinants of Health. Available at: https://www.who.int/social_determinants/en/ (accessed January 4, 2021).
- James, C. V. 2019. Actively Addressing Social Determinants of Health will Help Us Achieve Health Equity. CMS.gov Blog. Available at: https://www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity (accessed January 4, 2021).
- Baicker, K. and M. E. Chernew. 2017. Alternative Payment Models. JAMA Internal Medicine 177(2):222-223. https://doi.org/10.1001/jamainternmed.2016.8280.
- The MITRE Corporation. 2016. Accelerating and Aligning Population-Based Payment Models: Performance Measurement. Available at: https://hcp-lan.org/workproducts/pm-whitepaper-final.pdf (accessed January 4, 2021).
- Berwick, D. M. 1989. Continuous improvement as an ideal in health care. New England Journal of Medicine 320(1):53-56. https://doi.org/10.1056/NEJM198901053200110.
- Rentsch, C. T., F. Kidwai-Khan, J. P. Tate, L. S. Park, J. T. King Jr., M. Skanderson, R. G. Hauser, A. Schultze, C. I. Jarvis, M. Holodniy, V. Lo Re, K. M. Akgün, K. Crothers, T. H. Taddei, M. S. Freiberg, and A. C. Justice. 2020. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Medicine 17(9):e1003379. https://doi.org/10.1371/journal.pmed.1003379.
- Ioannou, G. N., E. Locke, P. Green, K. Berry, A. M. O’Hare, J. A. Shah, K. Crothers, M. C. Eastment, J. A. Dominitz, and V. S. Fan. 2020. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Network Open 3(9):e2022310. https://doi.org/10.1001/jamanetworkopen.2020.22310.
- Price-Haywood, E. G., J. Burton, D. Fort, and L. Seoane. 2020. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. New England Journal of Medicine 382:2534-2543. https://doi.org/10.1056/NEJMsa2011686.
- Jiménez, M. C., T. L. Cowger, L. E. Simon, M. Behn, N. Cassarino, and M. T. Bassett. 2020. Epidemiology of COVID-19 Among Incarcerated Individuals and Staff in Massachusetts Jails and Prisons. JAMA Network Open 3(8):e2018851. https://doi.org/10.1001/jamanetworkopen.2020.18851.
- Selden, T. M., and T. A. Berdahl. 2020. COVID-19 And Racial/Ethnic Disparities In Health Risk, Employment, And Household Composition. Health Affairs (Millwood) 39(9):1624-1632. https://doi.org/10.1377/hlthaff.2020.00897.
- Austin, J. M. and A. Kachalia. 2020. The State of Health Care Quality Measurement in the Era of COVID-19: The Importance of Doing Better. JAMA 324(4):333-334. https://doi.org/10.1001/jama.2020.11461.
- Hamlin, B., M. E. O’Kane, M. Barr, and P. Cotton. 2020. COVID-19 Underscores the Need for Digital Quality Measurement. Health Affairs Blog, August 26. https://doi.org/10.1377/hblog20200824.987845.
- Bui, D. P., I. See, E. M. Hesse, K. Varela, R. R. Harvey, E. M. August, A. Winquist, S. Mullins, S. McBee, E. Thomasson, and A. Atkins. 2020. Association Between CMS Quality Ratings and COVID-19 Outbreaks in Nursing Homes—West Virginia, March 17–June 11, 2020. Morbidity and Mortality Weekly Report 69(37):1300-1304. http://dx.doi.org/10.15585/mmwr.mm6937a5.
- Agency for Healthcare Research and Quality. n.d. About AHR’s Nursing Home COVID-19 Network. Available at: https://www.ahrq.gov/nursing-home/about/index.html (accessed January 4, 2020).
- Office of the Assistant Secretary for Planning and Evaluation. n.d. Background: Request from Congress for a Study of Social Risk Factors and Medicare’s Value-Based Purchasing Programs. Available at: https://aspe.hhs.gov/social-risk-factors-and-medicares-value-based-purchasing-programs (accessed January 4, 2021).
- Bogard, K., V. Murry, and C. Alexander, eds. 2017. Perspectives on health equity and social determinants of health. Washington, DC: National Academy of Medicine.
- National Academies of Sciences, Engineering, and Medicine. 2019. National Academies Report Helps Inform Metrics for Healthy People 2030. Press Release, August 6. Available at: https://www.nationalacademies.org/news/2019/08/national-academies-report-helps-inform-metrics-for-healthy-people-2030 (accessed January 4, 2021).
- Greenwood, B. N., R. R. Hardeman, L. Huang, and A. Sojourner. 2020. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proceedings of the National Academy of Sciences 117(35):21194-21200. https://doi.org/10.1073/pnas.1913405117.
- Agniel, D., S. C. Martino, Q. Burkhart, K. Hambarsoomian, N. Orr, M. K. Beckett, C. James, S. H. Scholle, S. Wilson-Frederick, J. Ng, and M. N. Elliott. 2019. Incentivizing Excellent Care to At-Risk Groups with a Health Equity Summary Score. Journal of General Internal Medicine. https://doi.org/10.1007/s11606-019-05473-x.
- Health Care Payment Learning & Action Network. n.d. What is the Health Care Payment Learning & Action Network? Available at: https://hcp-lan.org/ (accessed January 4, 2021).
- Institute of Medicine. 2003. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press. https://doi.org/10.17226/12875.
- Centers for Disease Control and Prevention. 2013. CDC Health Disparities and Inequalities Report—United States, 2013. Morbidity and Mortality Weekly Report 62(3). Available at: https://www.cdc.gov/mmwr/pdf/other/su6203.pdf (accessed January 4, 2021).
- Centers for Medicare and Medicaid Services. 2020. Preliminary Medicare COVID-19 Data Snapshot. Available at: https://www.cms.gov/files/document/medicare-covid-19-data-snapshot-fact-sheet-september2020.pdf (accessed January 4, 2021).
- Quinn, S. C., A. Jamison, J. An, V. S. Freimuth, G. R. Hancock and D. Musa. 2017. Breaking down the monolith: Understanding flu vaccine uptake among African Americans. SSM – Population Health 4:25-36. https://doi.org/10.1016/j.ssmph.2017.11.003.
- National Academies of Sciences, Engineering, and Medicine. 2020. Framework for Equitable Allocation of COVID-19 Vaccine. Washington, DC: The National Academies Press. https://doi.org/10.17226/25917.
- DeSalvo, K., B. Hughes, M. Bassett, G. Benjamin, M. Fraser, S. Galea, N. Garcia, and J. Howard. 2021. Public Health COVID-19 Impact Assessment: Lessons Learned and Compelling Needs. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202104c.
- Huges, D., and L. Zephyrin. 2020. Promoting Health Equity Through Accountable Communities for Health. Commonwealth Fund Blog. Available at: https://www.commonwealthfund.org/blog/2020/promoting-health-equity-through-accountable-communities-health (accessed July 16, 2021).
- Ogedegbe, G., J. Ravenell, S. Adhikari, M. Butler, T. Cook, F. Francois, E. Itturate, G. Jean-Lous, S. A. Jones, D. A. Onakomaiya, C. M. Petrilli, C. Pulgarin, S. Regan, H. Reynolds, A. Seixas, F. M. Volpicelli, and L. I. Horwitz. 2020. Assessment of Racial/Ethnic Disparities in Hospitalization and Mortality in Patients With COVID-19 in New York City. JAMA Network Open 3(12):e2026881. https://doi.org/ 10.1001/jamanetworkopen.2020.26881.
- Bilimoria, K. Y., J. D. Birkmeyer, H. Burstin, J. B. Dimick, K. E. Joynt Maddox, A. R. Dahlke, J. O. DeLancey, and P. J. Pronovost. 2019. Rating the Raters: An Evaluation of Publicly Reported Hospital Quality Rating Systems. NEJM Catalyst. https://doi.org/10.1056/CAT.19.0629.
- U.S. Centers for Disease Control (CDC). 2020. CDC COVID-19 Response Health Equity Strategy: Accelerating Progress Towards Reducing COVID-19 Disparities and Achieving Health Equity. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/cdc-strategy.html (accessed May 26, 2021).
- Quinn, S. C., A. Jamison, V. S. Freimuth, J. An, G. R. Hancock, and D. Musa. 2017. Exploring racial influences on flu vaccine attitudes and behavior: Results of a national survey of White and African American adults. Vaccine 35(8):1167-1174. https://doi.org/10.1016/j.vaccine.2016.12.046.
- Anderson, A. C., E. O’Rourke, M. H. Chin, N. A. Ponce, S. M. Bernheim, and H. Burstin. 2018. Promoting Health Equity and Eliminating Disparities Through Performance Measurement And Payment. Health Affairs 37:3: 371-377. https://doi.org/10.1377/hlthaff.2017.1301.
- National Academies of Sciences, Engineering, and Medicine. 2018. Engaging the Private-Sector Health Care System in Building Capacity to Respond to Threats to the Public’s Health and National Security: Proceedings of a Workshop. Washington, DC: The National Academies Press. https://doi.org/10.17226/25203.
- Centers for Medicare and Medicaid Services. 2020. Interim Final Rule Updating Requirements for Notification of Confirmed and Suspected COVID-19 Cases Among Residents and Staff in Nursing Homes. Available at: https://www.cms.gov/files/document/qso-20-29-nh.pdf (accessed May 26, 2021).
- Meyer, G. S., J. Battles, J. C. Hart, and N. Tang. 2003. The US Agency for Healthcare Research and Quality’s activities in patient safety research. International Journal for Quality in Health Care 15(Suppl 1):i25-30. https://doi.org/10.1093/intqhc/mzg068.
- Quality Interagency Coordination Task Force. 2000. Doing What Counts for Patient Safety: Federal Actions to Reduce Medical Errors and Their Impact: Summary of the Report of the Quality Interagency Coordination Task Force. Available at: https://repository.library.georgetown.edu/handle/10822/932907 (accessed May 26, 2021).
- Agency for Healthcare Quality and Research. n.d. AHRQ National Scorecard on Hospital-Acquired Conditions Updated Baseline Rates and Preliminary Results 2014-2017. Available at: https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/pfp/hacreport-2019.pdf (accessed May 26, 2021).
- U.S. Centers for Disease Control and Prevention (CDC). 2020. COVID-19 Long-Term Care Facility Guidance. Available at: https://www.cms.gov/files/document/4220-covid-19-long-term-care-facility-guidance.pdf (accessed May 26, 2021).
- Centers for Medicare & Medicaid Services. 2020. COVID-19 Nursing Home Dataset, Week of 5/24/20. Available at: https://data.cms.gov/Special-Programs-Initiatives-COVID-19-Nursing-Home/COVID-19-Nursing-Home-Dataset/s2uc-8wxp (accessed May 26, 2021).
- The Commonwealth Fund. 2020. Evidence from a Decade of Innovation: The Impact of the Payment and Delivery Systems Reform and the Affordable Care Act. The Commonwealth Fund. Available at: https://www.commonwealthfund.org/evidence-decade-innovation-impact-payment-and-delivery-system-reforms-affordable-care-act (accessed May 26, 2021).
- U.S. Congress, H.R. 6675 (89th). 1965. The Social Security Amendments of 1965. Available at: https://www.govtrack.us/congress/bills/89/hr6675/text (accessed May 26, 2021).
- Centers for Medicare & Medicaid Services. 2021. Quality Improvement Organizations. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityImprovementOrgs (accessed May 26, 2021).
- Centers for Medicare & Medicaid Services. n.d. The CMS Meaningful Measures Initiative: What It Means to Patients, Families, Clinicians and Providers. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Meaningful-Measures_What-It-Means-Fact-Sheet_508_2018-02-28.pdf (accessed May 26, 2021).
- Prang, K-H., R. Maritz, H. Sabanovic, D. Dunt, and M. Kelaher. 2021. Mechanisms and impact of public reporting on physicians and hospitals’ performance: A systematic review (2000-2020). PLoS One. https://doi.org/10.1371/journal.pone.0247297.
- Agency for Healthcare Research and Quality. 2019. AHRQ at 20. Available at: https://www.ahrq.gov/cpi/about/20timeline.html (accessed May 26, 2021).
- The Joint Commission. 2020. The Joint Commission suspends all regular surveys amid COVID-19 pandemic. Available at: https://www.jointcommission.org/resources/news-and-multimedia/news/2020/03/the-joint-commission-suspends-all-regular-surveys-amid–covid-19-pandemic/ (accessed May 31, 2021).
- The Joint Commission. 2020. Joint Commission International Resumes Accreditation Surveys. Available at: https://www.jointcommissioninternational.org/news-and-support/news/2020/06/joint-commission-international-resumes-accreditation-surveys/ (accessed May 31, 2021).
- Academy of General Dentistry. 2020. Response Guidance Update—20 November 2020. Available at: https://www.agd.org/docs/default-source/default-document-library/11202020_guidanceupdate.pdf?sfvrsn=260698bc_0 (accessed May 31, 2021).
- Center for Medicare and Medicaid Services. 2020. Clinical Laboratory Improvement Amendments (CLIA). Available at: https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA (accessed May 31, 2021).
- Government Accountability Office. 2020. Department of Health and Human Services, Centers for Medicare & Medicaid Services: Medicare and Medicaid Programs, Clinical Laboratory Improvement Amendments (CLIA), and Patient Protection and Affordable Care Act; Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency. Available at: https://www.gao.gov/products/b-332489?source=ra (accessed May 31, 2021).
- Center for Medicare and Medicaid Services. 2021. Toolkit On Covid-19 Vaccine: Health Insurance Issuers And Medicare Advantage Plans. Available at: https://www.cms.gov/files/document/COVID-19-toolkit-issuers-MA-plans.pdf (accessed May 31, 2021).
- Center for Medicare and Medicaid Services. 2021. Medicare COVID-19 Vaccine Shot Payment. Available at: https://www.cms.gov/medicare/covid-19/medicare-covid-19-vaccine-shot-payment (accessed May 31, 2021).
- Center for Medicare and Medicaid Services. 2021. Merit-based Incentive Payment System (MIPS) Participating in the Improvement Activities Performance Category in the 2021 Performance Year: Traditional MIPS. Available at: https://qpp-cm-prod-content.s3.amazonaws.com/uploads/1421/2021%20MIPS%20Improvement%20Activities%20User%20Guide.pdf (accessed May 31, 2021).
- The Joint Commission. n.d. Accreditation and Certification. Available at: https://www.jointcommission.org/accreditation-and-certification/ (accessed June 15, 2021).