Transforming the Economics of Clinical Trials
Introduction
Clinical trials are a means of gathering information about medical products or services. In medicine, however, they are also the fundamental means for advancing the underlying science and have enabled the many advances we have made in health care over the last few years. In many ways, clinical trials are the core infrastructure of the practice of medicine.
For an activity so vital to the field, the clinical research process is in serious trouble, especially in the United States. The cost of clinical trials for manufacturers of pharmaceuticals, biologics, and medical devices, as well as for public health investigators, continues to escalate. In pharmaceuticals (where this trend has been best documented), the costs have increased 7.4 percent annually over inflation for the last 20 years (DiMasi et al., 2003). The rising cost of clinical research is multifactorial but has been attributed to increased protocol complexity; an increase in local, regional, national, and international regulations/guidance and lack of harmonization among these; excessively risk-averse interpretations of regulations; and increasing time (and financial) pressures on clinician-investigators (Bollyky et al., 2010). Meanwhile, the need to conduct research in this environment has fueled the creation over the last three decades of a large and relatively new industry—clinical research organizations (CROs). While CROs improve efficiency for sponsors, their business practices also contribute to the current cost structure of clinical research. Irrespective of the factors driving the cost increases, the implications are clear. The greater the cost of research, the fewer new medical products that come to market, the less we know about the products that do, and the fewer initiated investigations of public health–inspired research questions. While we advance the concept of the “learning health care system” (Institute of Medicine [IOM], 2003), the cost of clinical research moves us further from this goal every day.
The Business of Clinical Trials
At its core, clinical research is not the practice of medicine but the collection of information about this practice. This conceptualization may provide some insight into the cost problem facing clinical research and could lead us to potential approaches for moving the clinical research effort onto a different cost trajectory. In other words, by viewing clinical research as an information and knowledge activity, we can look outside of medicine to understand the
economics of information-intensive businesses in the United States.
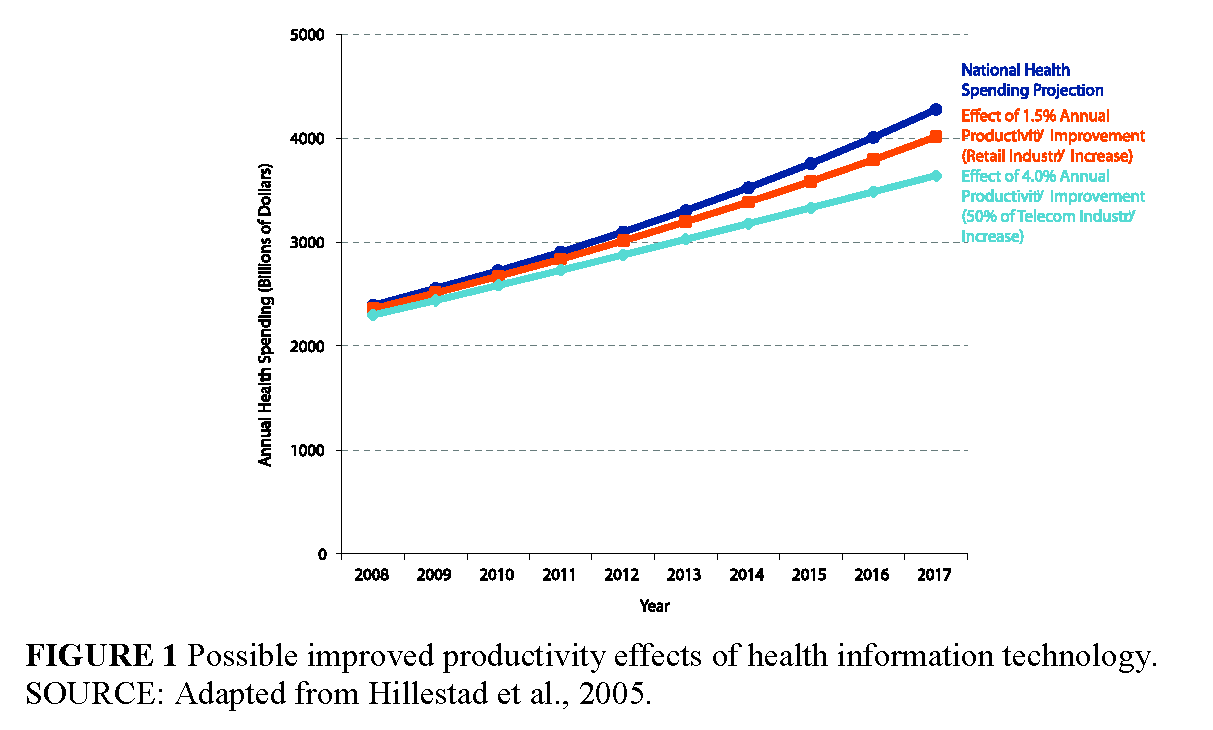
In the information technology world, for example, Gordon Moore—co-founder of Intel— famously predicted in 1965 that the number of chips on a “minimal cost” transistor would double every 2 years (Moore, 1965); this massive increase in performance of the microchip over time has led to the reciprocal effect of dramatically reducing the cost of computers since the first personal computer was introduced in 1981. We have seen similar economic gains over time in other information-intensive industries, such as telecommunications (see Figure 1, p. 7, for an estimate of improved productivity effects of health information technology) (Hillestad et al., 2005). The trajectory of cost in these information industries is driven by changes in both the technology and the underlying business models supporting the industry. Understanding this process is critical to our understanding of ways in which we can address the underlying cost issues in clinical research.
In his classic book The Innovator’s Dilemma, Clay Christensen describes how new technology is brought to market by new companies through new business models, leading to cost and quality improvements over time (Christensen, 1997). He describes how cost and quality improvements in the computer disk–drive industry were driven by this creative destruction process in the marketplace. In re-conceptualizing clinical research as the development of information about the practice of medicine, we can begin to lay out a plan for the transformation of the economics of clinical research that could mirror the cost trajectories found in other information industries. There are two components of this model—the core technology for clinical research and the core business model for clinical research.
A decade ago, clinical research relied largely on paper-based technology, in which site investigators would record their data on special, multipart case report forms (CRFs). These forms were collected centrally, entered into a database, and analyzed. The business process around clinical research entailed efforts to support this system to ensure that the data collected were accurate and valid. The cost of this model was related to the tremendous amount of labor involved in this exercise. Other costs were related to patient screening and recruitment to the trials.
Globalization of clinical research has evolved in part in response to the high unit costs of clinical research in developed economies, but it has not enabled the development of a different business model for clinical research (Glickman et al., 2009). Over the last decade, electronic data capture (EDC) has made some progress in transforming this model. In an EDC-enabled environment, paper forms are replaced by electronic forms, whereby sites enter their data into an electronic database. This technology has produced some efficiency gains as edit-checks reduce the cost of the manual data-query process. However, the EDC platform was largely embedded in the traditional clinical research business model, with legacy concepts around site monitoring and validation. New opportunities for central statistical monitoring of data in these systems were not widely embraced (Morrison et al., 2011). Patient screening and recruitment costs remained largely unchanged.
Over the next few years, providers will adopt electronic health records (EHRs) as a means of capturing data that are also critical to the clinical research process. Informatics can provide data-capture tools to create structured data forms in the medical records for research protocols, as well as data tools to allow aggregation of data across research sites. Because we can search clinical data repositories electronically to find eligible patients for research, for the first time EHR-enabled clinical research has the potential to reduce site-screening and recruitment costs of clinical research. The business framework for this type of clinical research enterprise has yet to be established.
A concurrent or subsequent research technology will be mobile-based technology that will allow direct contact with research subjects and direct data collection from patients. This technology could be paired with traditional clinical research processes or could enable a new model of direct-to-patient research efforts. The economics of this latter model of clinical research could be a log order less than current research costs.
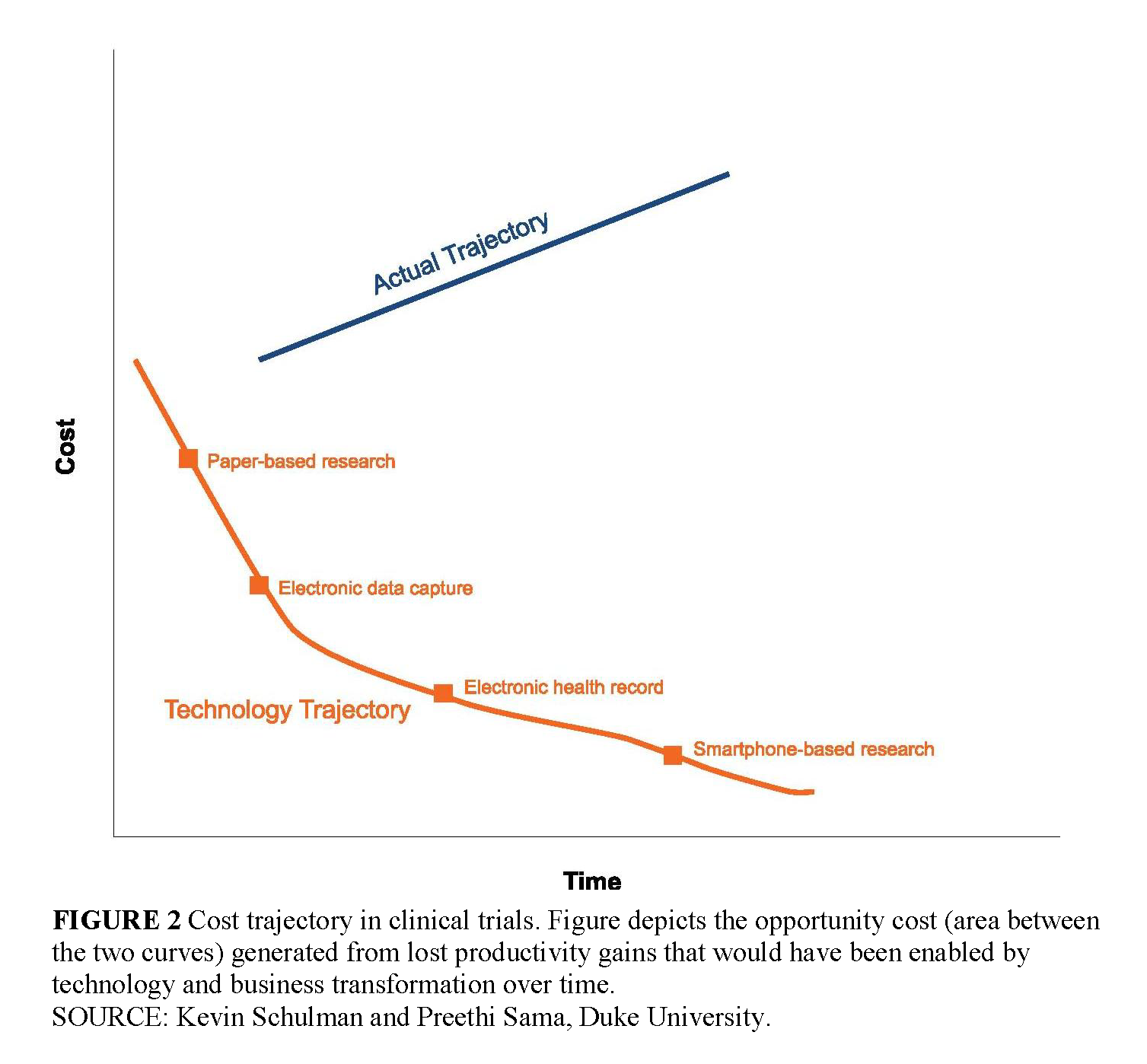
Viewed in the timeframe of technology-enabled business models, we can make some general statements about the development of clinical research as an industry. While the technology for gathering clinical data for research has evolved over time, the business model supporting this technology clearly has not evolved with each stage of technology transformation. As a result, the potential economic opportunities from technology have been absorbed by legacy costs and processes shaped by previous generations of the business model for clinical research. These legacy costs exist in the sponsor organizations, in the regulatory environment, in the provider environment, and in the clinical research organizations themselves. In this context, innovation in technology has not generated the potential economic benefits that follow from business transformation. Further, hidden in this structure is the tremendous cost to sponsors of non–value-added processes within the clinical research environment that add tremendously to the cost of clinical research (Dilts and Sandler, 2006; DiMasi et al., 2003). The tremendous opportunity cost generated by lost productivity gains that would have been enabled by technology and business transformation over this time period is illustrated in Figure 2 (p. 8).
Transforming the Economics of Clinical Trials
This framework provides some insight into potential paths forward for the clinical research community. It implies an agenda of enabling business transformation with each generation of technology, requiring the retirement of outmoded business processes, and models to achieve economic transformation of clinical research. While this is a natural process in unregulated environments, this effort would need to take the form of a dedicated discipline in a regulated environment (Curtis and Schulman, 2006).
As we consider approaches to enable business transformation that will keep pace with new technology and the escalating need for information about the practice of medicine, it would be wise to first articulate a common goal for the clinical research enterprise: to facilitate the efficient conduct of high-quality clinical trials to address pressing medical questions and evaluate therapeutic interventions. This goal serves both public health and business interests. In short, we want to do more trials at lower cost, complete them faster, and enhance their quality.
Engaging all sectors affected by clinical research in the conceptualization of new business models should facilitate the adoption of those new models. Although all sectors would likely support the overarching goal stated above, local concerns and incentives within individual sectors may prioritize maintenance of certain aspects of the existing business model over transformation of the research model. For the pharmaceutical, biological, and device industries, one might ask, “Why would a pharmaceutical company persist in conducting and funding clinical research activities that add cost but not value as measured by increased patient safety or reliability of data?” Understanding the lens through which actors assess the opportunity for business transformation will be critical to the success of this effort. Some suggest that, within the pharmaceutical industry, maintenance of activities that do not add value is not due to misinformation about the value of the activity but rather to avoidance of perceived regulatory risk—e.g., concern that a regulatory audit at a clinical site might delay or prevent approval of a product under development (which has a much greater cost to the sponsor) (Bollyky et al., 2010).
As technology evolves and industry sponsors, academicians, or the regulators themselves identify practices and guidelines based on outmoded business processes, collaborative initiatives such as the Sensible Guidelines group (Yusuf et al., 2008), the IOM Forum on Drug Discovery, Development, and Translation and the Clinical Trials Transformation Initiative (CTTI) (For more information on the projects of the Clinical Trials Transformation Initiative, visit https://www.ctticlinicaltrials.org) can engage regulators, industry, government researchers, academicians, and patients in conceptualizing and facilitating new business models. Active engagement to identify and overcome these issues will be critical in the regulated clinical research environment. Regulators can also help by clarifying any misperceptions of the current regulatory position, thereby providing reassurance to those afraid of migrating from the existing business model. Given the risks of business transformation for industry, these clarifications need to be as formal and timely as possible.
A recent example of such an approach to clinical trial monitoring is encouraging and may provide a prototype for a collaborative solution in the regulated environment. The cost of site management (monitoring) of clinical trials has been estimated to represent 25 to 30 percent of the cost of a clinical trial (Eisenstein et al., 2005). In a recent survey of 65 organizations that conduct or sponsor clinical trials, over 80 percent of responding pharmaceutical, biological, and device companies indicated that they always perform on-site monitoring visits and always perform source-document verification at the site. Despite over 80 percent of those companies reporting access to centrally available data to evaluate site performance, less than a quarter of them always used a centralized monitoring process to guide, target, or supplement site visits (Morrison et al., 2011). Despite a widespread perception that it is the regulators who require 100 percent source-document verification of all data items to ensure quality, a recent reflection paper from the European Medicines Agency (EMA) on risk-based quality management in clinical trials (EMA, 2011) and Food and Drug Administration (FDA) guidance for industry on a risk-based approach to monitoring (FDA, 2011) both clearly state otherwise. These regulatory documents acknowledged findings of the Sensible Guidelines group, CTTI, and other collaborative initiatives that influenced their recommendation of a risk-based solution to trial oversight. Both the EMA paper and the FDA’s guidance embrace the use of centralized monitoring through real-time advanced data systems, consistent with the advantages of new technology.
The recent announcement by Pfizer of a clinical trial of a marketed product that will be conducted completely over the Internet (i.e., without identified clinical sites) was remarkable for the fact that the sponsor and the FDA worked very closely with the principal investigator to design and test this new approach for reliability and safety (Mansell, 2011). This model suggests an alternative pathway that can provide a more timely response to sponsors to address questions about new research business models.
It would be worthwhile also to consider the local cultural and financial incentives of academic and government institutions that engage in clinical research and clinical care. If clinical research is the collection of information about the practice of medicine, new technology and business models that promote efficiency should enable many more clinical questions to be addressed and enable more potential savings by identifying expensive but ineffective
interventions. It is striking that the health care system has developed so that its business model can conflict financially with improvements in the practice of medicine. For example, many institutions pursued aggressive programs for high-dose chemotherapy and stem-cell transplantation for breast cancer before the definitive clinical evidence was developed indicating lack of efficacy of this treatment for breast cancer (Schulman et al., 2003). Leadership at the institutional level must ensure that the financial interests of the local institution do not overpower the public health interest in generating evidence to drive the practice of medicine.
There is also an issue of “sunk costs” from the perspective of an institution, or decision making guided by past investment strategies in clinical research. For instance, once an academic institution has invested in creating a smoothly functioning institutional review board (IRB) and an electronic system for protocol submissions to the IRB, officials at that institution may be reluctant to defer to central IRBs for industry-sponsored multicenter trials being conducted at their institution due to concern about the loss of financial support for their own infrastructure. While these concerns are valid, business transformation requires the discipline to jettison these investments when better opportunities arise. Obviously, this discipline for creative destruction of old models is not a core competency within the health care system. New business models may be thwarted if these concerns are not directly addressed.
An overall approach to ensure that the business model keeps pace with new technology and information needs should start with an agenda for research on how we do research. This agenda should be dedicated to addressing the potential issues in business transformation with each stage of technology migration. This effort requires a clear statement of the goals of different steps in the research process and an understanding of the regulatory and business processes that support each of these steps. The research agenda will then review the potential transformation of these steps given the next generation of technology. This could include recasting goals, transforming regulations, and transforming business processes. The goals of this agenda should be forward-looking rather than focused on refining the current environment. This agenda should be driven by a goal of transforming the clinical research environment every 2 to 5 years, which will lead to tremendous improvements in the economic efficiency of clinical research.
While the research will highlight pathways to business-process transformation, implementing this collaborative approach to business-process improvement is antithetical to the usual business transformation. Incumbent business models inherently fight business-process transformation when given the opportunity, either consciously or unconsciously. Experience is used to argue for the current business model with regulators, and, in the absence of organizations representing business transformation, this acts as a potent force. The concept of a forward-looking research agenda is one means of mitigating this natural response of incumbent organizations. Dedicating part of the regulatory structure to support this agenda would be another means of advancing this goal (i.e., safe harbors for business-process innovation could be a critical enabling strategy). Organizing the clinical community to enable these changes would also be a critical step in this process (e.g., business transformation could include removing clinicians or nurses from some of their current roles in the research process).
Although clinical research clearly provides information that underpins the practice of medicine, more education about clinical research is needed for health professionals. A recent report on the ClinicalTrials.gov results database raised concerns about the quality of some submitted entries, which may relate directly to insufficient knowledge on the part of investigators about fundamental precepts of clinical research—e.g., 20 percent of trials with recorded results reported more than two primary outcome measures, and 5 percent of trials reported more than five primary outcome measures (Zarin et al., 2011). Education about clinical research is necessary to ensure the ability to develop well-designed clinical research protocols, to maximize practitioners’ and patients’ participation in trials, to produce clinicians capable of competently interpreting and applying the results of completed trials, and to help facilitate new models of clinical research. These skills should become part of essential knowledge of the clinical care community and the core of the learning health care system. Health care professionals trained in clinical research can more competently analyze and streamline complex regulatory and administrative processes for clinical research within institutions to facilitate the adoption of new business models.
Conclusion
The economic crisis in clinical research is driven by the inability to transform the business model for research as the core informatics technology evolves. A concerted, multistakeholder effort to address adoption of new business models for clinical research is a critical step in unlocking the potential economic transformation of clinical research in the coming decade.
References
- Bollyky, T. J., I. M. Cockburn, and E. Berndt. 2010. Bridging the gap: Improving clinical development and the regulatory pathways for health products for neglected diseases. Clinical Trials 7:719-734. https://doi.org/10.1177/1740774510386390
- Christensen, C. M. 1997. The Innovator’s Dilemma. Cambridge, MA: Harvard Business Press.
- Curtis, L. H., and K. A. Schulman. 2006. Overregulation of health care: Musings on disruptive innovation theory. Law and Contemporary Problems 69:195-206. Available at: https://scholarship.law.duke.edu/lcp/vol69/iss4/9
- Dilts, D. M., and A. B. Sandler. 2006. Invisible barriers to clinical trials: The impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. Journal of Clinical Oncology 24:4545-4552. https://doi.org/10.1200/JCO.2005.05.0104.
- DiMasi, J.A., R. W. Hansen, and H. G. Grabowski. 2003. The price of innovation: New estimates of drug development costs. Journal of Health Economics 22:151-185. https://doi.org/10.1016/S0167-6296(02)00126-1.
- Eisenstein, E. L., P. W. Lemons II, B. E. Tardiff, K. A. Schulman, M. K. Jolly, and R. M. Califf. 2005. Reducing the costs of phase III cardiovascular clinical trials. American Heart Journal 149:482-488. https://doi.org/10.1016/j.ahj.2004.04.049.
- EMA (European Medicines Agency). 2011. Reflection Paper on Risk-Based Quality Management in Clinical Trials. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500110059.pdf (accessed October 2, 2011).
- FDA (Food and Drug Administration). 2011. Guidance for Industry Oversight of Clinical Investigations—A Risk-Based Approach to Monitoring [Draft]. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM269919.pdf (accessed October 2, 2011).
- Glickman, S. W., J. G. McHutchison, E. D. Peterson, C. B. Cairns, R. A. Harrington, R. M. Califf, and K. A. Schulman. 2009. Ethical and scientific implications of the globalization of clinical research. New England Journal of Medicine 360:816-823. https://doi.org/10.1056/NEJMsb0803929.
- Hillestad, R., J. Bigelow, A. Bower, F. Girosi, R. Meili, R. Scoville, and R. Taylor. 2005. Can electronic medical record system transform health care? Potential health benefits, savings, and costs. Health Affairs 24:1103-1117. https://doi.org/10.1377/hlthaff.24.5.1103.
- IOM (Institute of Medicine). 2007. The Learning Healthcare System: Workshop Summary. Washington, DC: The National Academies Press. https://doi.org/10.17226/11903.
- Mansell, P. 2011. Pfizer announces “virtual” clinical trial pilot in US. Pharma Times Online, June 9. http://www.pharmatimes.com/article/11-06-09/Pfizer_announces_virtual_clinical_trial_pilot_in_US.aspx. (accessed September 12, 2011).
- Moore, G. 1965. Cramming more components onto integrated circuits. Electronics Magazine, April 19.
- Morrison, B. W., C. J. Cochran, J. G. White, J. Harley, C. F. Kleppinger, A. Liu, J. T. Mitchel, D. F. Nickerson, C. R. Zacharias, J. M. Kramer, and J. D. Neaton. 2011. Monitoring the quality of conduct of clinical trials: A survey of current practices. Clinical Trials 8:342-349. https://doi.org/10.1177/1740774511402703.
- Schulman, K. A., E. A. Stadtmauer, S. D. Reed, H. A. Glick, L. J. Goldstein, J. M. Pines, J. A. Jackman, S. Suzuki, M. J. Styler, P. A. Crilley, T. R. Klumpp, K. F. Mangan, and J. H. Glick. 2003. Economic analysis of conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. Bone Marrow Transplant 31:205-210. https://doi.org/10.1038/sj.bmt.1703795.
- Yusuf, S., J. Bosch, P. J. Devereaux, R. Collins, C. Baigent, C. Granger, R. Califf, and R. Temple. 2008. Sensible guidelines for the conduct of large randomized trials. Clinical Trials 5:38-39. https://doi.org/10.1177/1740774507088099
- Zarin, D. A., T. Tse, R. J. Williams, R. M. Califf, and N. C. Ide. 2011. The ClinicalTrials.gov results database: Update and key issues. New England Journal of Medicine 364:852-860. https://doi.org/10.1056/NEJMsa1012065.