Integrating United States Biomanufacturing Infrastructure across Vaccines and Therapeutics
 The National Academies are responding to the COVID-19 pandemic.
The National Academies are responding to the COVID-19 pandemic.
Visit our resource center >>
In 2021, as the majority of the world’s population eagerly waits to receive safe and effective COVID-19 vaccines, factories worldwide are producing bits of the vaccine components—mRNA, lipids, proteins, key reagents, and attenuated viruses—and then assembling them into complete, ready-to-inject vaccines. This process has now gained significant attention, not just for the speed at which the scientific community innovated and developed multiple and hugely effective vaccines for COVID-19, but also because of the massive shortfalls in vaccine production, supply, and equitable distribution across the world. Also under scrutiny is the level of government intervention that has been necessary to accelerate vaccine production and distribution. What is less recognized is that such inadequacies in biomanufacturing and supply chain robustness are not just limited to vaccines. The problems are systemic, plaguing all emerging biologics-based therapies—from cell and gene therapies (CGTs), engineered tissues and organoids (ETOs), to next-generation vaccines. Similar to the COVID-19 vaccines, thousands of patients are waiting for cell therapies to treat their cancer, many more are waiting for gene therapies to address their inherited disease, and only a privileged few can access these therapies. The lack of appropriate advanced biomanufacturing and supply-chain infrastructure is a key barrier for widespread and equitable access of these emerging therapies and requires a fundamental change in national and global science policy.
Just as factories produce components of the COVID-19 vaccines, factories also make the constituent DNA, RNA, proteins, viruses, cells, and tissues for advanced therapies. Short supply and delays in key reagents, especially during a pandemic and as demand increases rapidly, can stifle the CGT, ETO, and emerging vaccine fields. Similarly, distribution logistics of these complex and temperature-sensitive therapeutics deserve serious attention, and appropriate infrastructure to ensure scalable, smooth, timely, and efficient access is critically needed. In addition to supply chain and logistics, advances in the science, technology, and infrastructure for biomanufacturing (a scientific field focused on making various biological products efficiently, in high-quality, and at scale [1,2,3,4]), is necessary to improve the supply, consistency, quality, cost, and access of products to meet the current and future demand worldwide.
In light of these similarities, preparation for the world’s post-pandemic future should consider the growth of the vaccine and advanced therapies fields together. Recent scale-up and modernization of vaccine biomanufacturing will have spillover effects for CGTs, while much-needed future innovations and investments in CGT and ETO manufacturing will provide surge and advanced manufacturing capacity for vaccines. While some CGTs are in development for infectious diseases like COVID-19, the primary targets of CGTs are cancer, blood disorders, liver diseases, and numerous rare disorders [5]. Compared to communicable diseases, the conditions addressed by CGT are less cyclical or episodic, resulting in a more linear, plateauing, and predictable demand for CGT. As the need for worldwide, immediate supply of a particular vaccine wanes, a common, integrated biomanufacturing infrastructure and framework could be used for both advanced therapies and emerging vaccines.
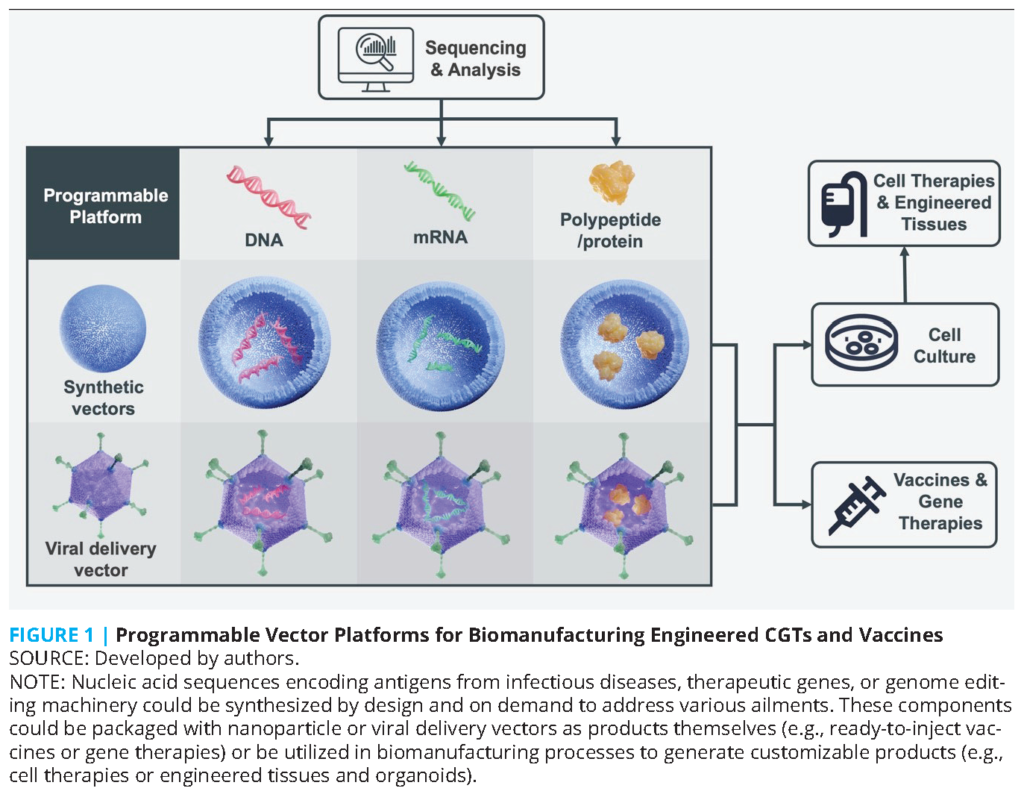
Even before the COVID-19 pandemic, high demand and short supply were common features of next-generation CGTs and vaccines. For example, there were year-long queues at biomanufacturing facilities to make clinical-grade viral vectors from 2017 onwards [6]. The active ingredients in many CGTs also contain RNAs, proteins, and viral vectors, as in vaccines (see Figure 1). Vectors (e.g., adenovirus) and lipids (e.g., 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]) could carry therapeutic genes for gene therapy or encode antigens for vaccines. Plus, many of the plasmids, producer cell lines, cell-free systems, in vitro transcription enzymes, and recombinant proteins used in biomanufacturing are common, as are the analytical instruments measuring these components [1,2,3,4]. Both CGTs and vaccines leverage advances in programmable biology by modifying the nucleic acids within the manufacturing process or product. Today’s processes build on decades of steady innovation in gene synthesis, genomic sequencing, genetic engineering, editing genomes, and synthetic biology [7]. Overall, biomanufacturing processes for CGTs and vaccines use common knowledge, technologies, and workforce that are potentially applicable to both sectors. In addition, current and future data science, artificial intelligence (AI), supply chain, and cyberinfrastructure innovations in biomanufacturing can impact CGTs and vaccines alike.
Customizable and modular platforms now provide unprecedented flexibility to develop new vaccines for evolving viruses and tailored therapeutics to treat disparate disease targets across diverse populations. For example, BioNTech initially targeted cancer with its technology platform and was able to pivot to COVID-19 vaccines in 2020. The rapid development and emergency use authorizations in 2020 in the U.S. of vaccines from Moderna and Pfizer-BioNTech (and perhaps Novavax later in 2021), as well as the role that government policies have played in ramping up vaccine production, purchase, distribution, and administration, illustrate this technology’s promise and capabilities. Many CGTs currently in development can be customized to treat hundreds of rare diseases and cancers. However, despite multibillion-dollar markets with recent double-digit annual growth for both vaccines and CGTs, biomanufacturing efforts remain siloed and balkanized, mainly in the private sector within companies focused on either field. Stronger connections across these two fields—and with adjacent scientific, technological, clinical, infrastructure, and policy fields—would increase resiliency and preparedness to meet the increasing demand for these products. Specifically, additional work in the following five directions (see Figure 2) could build a more integrative approach to biomanufacturing across vaccines and CGTs.
Priorities for Building an Integrative Approach to Biomanufacturing
Customizable Platforms
The complexity of biological products relative to traditional small molecule drugs present multifaceted challenges for regulatory science, and therefore stringent regulatory oversight (or good manufacturing practice) is required for biomanufactured products. For example, a simple switch of vendors moving from a bovine to a pig serum can change the sugars on the surface of proteins and modify the immune response to the product, thereby prompting additional regulatory scrutiny. Hence, drug master files on qualified tools, reagents, and vectors provide essential data for sponsors to cross-reference in their applications for emergency authorizations. Such an approach ultimately streamlines review by regulatory authorities of applications by sponsors, and there is a greater need for more common and customizable tools, reagents, and vectors for the modular, programmable platforms used in vaccines and CGT biomanufacturing (see Figure 1). Efforts like Platform Vector Gene Therapy [6], Coalition for Epidemic Preparedness Innovations’ (CEPI) platform technologies for Disease X, and proprietary platforms at Moderna [8] provide some examples, and further integration of technology, best practices, and standardization efforts across both CGT and vaccine product development is possible.
Flexible Manufacturing
At any point in time, disease burden can be highly heterogeneous and local. Even within a population in a specific locale, disparities across race, ethnicity, and socioeconomic status can lead to variable demands for different vaccines and CGTs. Further, viral variants can cluster in a region and sweep heterogeneously across populations and geographies. A flexible manufacturing approach could help facilities quickly pivot from centralized manufacturing of a single product to regional or point-of-care production of different products. In the United States, if the East and West Coasts surge in COVID-19 cases and lockdowns, facilities in the Midwest could ramp up production—and vice versa. Resilience within this interconnected infrastructure could also be enhanced with flexible biomanufacturing. A key aspect of this emerging concept is a shift away from the traditional “process is the product” concept and toward a reliance on product critical quality attributes (CQAs) (i.e., the range of specific product properties within which the product performs in a predictive manner in a certain disease and patient). This shift, combined with “intelligent automation” where sensors and in-process or at-line measurements of quality attributes allow one to control process parameters and keep CGT or vaccine products within a defined quality, will allow for reproducible and predictive outcome in patients and reduce cost and need for large-scale production. Also, the logistics of supply chain robustness and storage, shipping, and delivery of raw materials and finished products are crucial since delays in any part of this supply chain can propagate [9] and eventually lead to problematic consequences in delaying vaccinations or treatment. Modeling an integrative biomanufacturing system from needle to needle while optimizing quality, scale, cost, resources, and supply could reduce the frequency of batch failures, anticipate and eliminate bottlenecks and weak points, and deliver products more efficiently, as recently demonstrated in CGT manufacturing [10]. Rapid vaccine response efforts at CEPI and Gingko Bioworks are examples of flexible manufacturing approaches that support the production of many different types of products from common source materials.
Data Science, AI, and Cyberinfrastructure
A fragmented digital and cyberinfrastructure is a poor way to track health outcomes for an increasingly mobile population. For CGT, long-term monitoring for adverse events is already required by regulatory authorities—in some cases, up to 15 years—since adverse events like cancer from genetic modifications could take years to develop after treatment [11]. Regulatory agencies also recommend extended monitoring of vaccinated individuals over months to years. With an integrated data infrastructure across biomanufacturing and clinical outcomes, data science and AI concepts could provide new insights into how to improve processes, as formulation differences and dosing could be correlated to patient outcomes in electronic health records and long-term follow-up studies [18]. Intense pressures on biomanufacturing during emergencies can cause inadvertent changes in formulations, as seen in the Oxford/AstraZeneca COVID-19 vaccine trials [12]. Data from these studies and participants should not be entirely discarded but should be used within a digital infrastructure to inform future studies. The UK Biobank and UC Health system are examples of integrated clinical data infrastructures for extended, longitudinal monitoring. These and many similar efforts currently lack a strong integration of manufacturing information on the products administered to patients, although this challenge is being tackled in budding efforts in the United States like the NSF Engineering Research Center for Cell Manufacturing Technologies (CMaT) and the Marcus Center for Therapeutic Cell Characterization and Manufacturing (MC3M). For example, big data analytics and AI tools are being used to understand and identify CQAs and the corresponding critical process parameters that control those CQAs during manufacturing. Similarly, AI tools are being used to find correlative biomarkers that are predictive of patient outcomes for a certain product and disease, which can then be controlled and monitored during manufacturing to yield consistent, high-quality products [13]. Such approaches, combined with supply chain and logistics modeling (i.e., an end-to-end data-driven manufacturing infrastructure) could be transformative to both the CGT and vaccine fields.
Data Sharing and Partnering Across the Public-Private Continuum
Biomanufactured products can be quintessential public goods (e.g., vaccines) or the ultimate bespoke therapy tailored for an ultrarare patient (e.g., “n of 1” therapies [14]). Incentives that enable flexibility and interconnection to easily navigate these markets and business models would lead to a public-private infrastructure that responds rapidly to variable demands for both types of products. CEPI and Catapult in the United Kingdom, with the Centre for Commercialization of Regenerative Medicine, the National Institute for Innovation in Manufacturing Biopharmaceuticals, the Advanced Regenerative Manufacturing Institute, CMaT, and MC3M in North America, have all gained significant momentum. These efforts could take further advantage of a sharing and collaborative spirit shown in the scientific community in 2020, as demonstrated in the surge in the circulation of preprints and sequences [15].
New public-private partnerships are also needed to keep the world prepared and stocked, especially to develop the next generation of data-driven biomanufacturing infrastructure that combines fundamental research with technology development in flexible manufacturing, data science, supply chain optimization, and feedback-driven biomanufacturing, as discussed earlier. The US Strategic National Stockpile of therapeutics [16] in the event of a nuclear or chemical attack is an example of partnering across this public-private divide. Similar preparedness and urgency could address less punctuated but nonetheless urgent demands for CGTs and vaccines. Preordering products and stockpiling is part of a broader strategy to provide increased access to capital, knowledge, and intellectual property by state and federal governments. These actions are all necessary, and given sufficient political will, they could have further energy if organized under a large effort like the Manhattan Project or moonshot solution for biomanufacturing. In the United States, the Cancer Moonshot Initiative is an antecedent of future accelerated efforts, potentially within a recently-proposed Advanced Research Projects Agency-Health (ARPA-H) [17].
Workforce Training
Finally, in addition to traditional capital investment, the biomanufacturing field needs investment in human capital. Besides keeping up with the latest biology and engineering, the technicians and leadership in biomanufacturing need to understand and use new advances in automation and data science [1]. The biomanufacturing workforce has now been pressure tested, as they have kept operations up and running despite lockdowns and social distancing requirements during the COVID-19 pandemic. For example, many biomanufacturing organizations now have an updated list of essential people who have established routines and protocols to keep biomanufacturing facilities operating in the event of another pandemic-related lockdown. The professional development of these people should be nurtured, so that the wisdom of working through a pandemic is preserved within the workforce. In addition to a national stockpile of biomanufactured vaccines or therapeutics, a “national guard” for biomanufacturing could be just as important. Such a workforce needs to come not just from four-year colleges and universities and post-graduate trainees, but also, importantly, from the nation’s two-year technical and community colleges, which are the primary supplier of manufacturing technicians across all industries. In April 2020, students in the life sciences quickly organized to establish a COVID-19 National Scientist Volunteer Database to fill in during shortages at facilities, and such efforts building on a public service ethos could be given more support to increase our preparedness.
Conclusion
In summary, the COVID-19 pandemic is a portal into the current state of our biomanufacturing infrastructure—illuminating opportunities to strategically invest for greater preparedness. As the demand for vaccines and therapeutics increases, a concomitant strengthening of biomanufacturing infrastructure requires new cross-cutting integration across the vaccines and CGT fields to meet today’s needs, and in due course, the post-pandemic world.
Join the conversation!
![]() Tweet this! #COVID19 has made the world pay attention to vaccine development and production. Authors of a new #NAMPerspectives commentary urge continued focus on reinforcing biomanufacturing and supply chain robustness moving forward: https://doi.org/10.31478/202104e
Tweet this! #COVID19 has made the world pay attention to vaccine development and production. Authors of a new #NAMPerspectives commentary urge continued focus on reinforcing biomanufacturing and supply chain robustness moving forward: https://doi.org/10.31478/202104e
![]() Tweet this! “The lack of advanced biomanufacturing and supply chain infrastructure is a key barrier for widespread and equitable access to emerging therapies.” Authors of a new #NAMPerspectives commentary call for integration of a fragmented system: https://doi.org/10.31478/202104e
Tweet this! “The lack of advanced biomanufacturing and supply chain infrastructure is a key barrier for widespread and equitable access to emerging therapies.” Authors of a new #NAMPerspectives commentary call for integration of a fragmented system: https://doi.org/10.31478/202104e
![]() Tweet this! Authors of a new #NAMPerspectives commentary posit that vaccine development and distribution should be considered alongside similar processes for advanced therapies, as the two can complement and reinforce one another: https://doi.org/10.31478/202104e
Tweet this! Authors of a new #NAMPerspectives commentary posit that vaccine development and distribution should be considered alongside similar processes for advanced therapies, as the two can complement and reinforce one another: https://doi.org/10.31478/202104e
Download the graphics below and share them on social media!
References
- Roh, K.-H., R. M. Nerem, and K. Roy. 2016. Biomanufacturing of Therapeutic Cells: State of the Art, Current Challenges, and Future Perspectives. Annual Review of Chemical and Biomolecular Engineering 7:455-478. https://doi.org/10.1146/annurev-chembioeng-080615-033559.
- Aijaz, A., M. Li, D. Smith, D. Khong, C. LeBlon, O. S. Fenton, R. M. Olabisi, S. Libutti, J. Tischfield, M. V. Maus, R. Deans, R. N. Barcia, D. G. Anderson, J. Ritz, R. Preti, and B. Parekkadan. 2018. Biomanufacturing for clinically advanced cell therapies. Nature Biomedical Engineering 2:362-376. https://doi.org/10.1038/s41551-018-0246-6.
- Bundy, B. C., J. P. Hunt, M. C. Jewett, J. R. Swartz, D. W. Wood, D. D. Frey, and G. Rao. 2018. Cell-free biomanufacturing. Current Opinion in Chemical Engineering 22:177-183. https://doi.org/10.1016/j.coche.2018.10.003.
- Kis, K., R. Shattock, N. Shah, and C. Kontoravdi. 2019. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnology Journal 14(1):e1800376. https://doi.org/10.1002/biot.201800376.
- Haddock, R., S. Lin-Gibson, N. Lumelsky, R. McFarland, K. Roy, K. Saha, J. Zhang, and C. Zylberberg. 2017. Manufacturing Cell Therapies: The Paradigm Shift in Health Care of This Century. NAM Perspectives. Discussion Paper. National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201706c.
- Brooks, P. J., E. A. Ottinger, D. Portero, R. M. Lomash, A. Alimardanov, P. Terse, X. Xu, R. J. Chandler, J. Geist Hauserman, E. Esposito, C. G. Bönnemann, C. P. Venditti, C. P. Austin, A. Pariser, and D. C. Lo. 2020. The Platform Vector Gene Therapies Project: Increasing the Efficiency of Adeno-Associated Virus Gene Therapy Clinical Trial Startup. Human Gene Therapy 31(19-20):1034-1042. https://doi.org/10.1089/hum.2020.259.
- Tan, X., J. H. Letendre, J. J. Collins, and W. W. Wong. 2021. Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics. Cell 184(4):881-898. https://doi.org/10.1016/j.cell.2021.01.017.
- Widge, A. T., N. G. Rouphael, L. A. Jackson, E. J. Anderson, P. C. Roberts, M. Makhene, J. D. Chappell, M. R. Denison, L. J. Stevens, A. J. Pruijssers, A. B. McDermott, B. Flach, B. C. Lin, N. A. Doria-Rose, S. O’Dell, S. D. Schmidt, K. M. Neuzil, H. Bennett, B. Leav, M. Makowski, J. Albert, K. Cross, V.-V. Edara, K. Floyd, M. S. Suthar, W. Buchanan, C. J. Luke, J. E. Ledgerwood, J. R. Mascola, B. S. Graham, J. H. Beigel, and mRNA-1273 Study Group. 2021. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. New England Journal of Medicine 384(1):80-82. https://doi.org/10.1056/NEJMc2032195.
- Wang, K., Y. Liu, J. Li, C. White, B. Wang, and B. L. Levine. 2020. Modeling the Effects of Supply Chain and Operator Disruptions on Cell Therapy Manufacturing Facility Operations During the COVID-19 Pandemic. American Pharmaceutical Review. Available at: https://www.americanpharmaceuticalreview.com/Featured-Articles/567500-Modeling-the-Effects-of-Supply-Chain-and-Operator-Disruptions-on-Cell-Therapy-Manufacturing-Facility-Operations-During-the-COVID-19-Pandemic/ (accessed April 13, 2021).
- Wang, K., Y. Liu, J. Li, B. Wang, R. Bishop, C. White, A. Das, A. D. Levine, L. Ho, B. L. Levine, and A. D. Fesnak. 2019. A multiscale simulation framework for the manufacturing facility and supply chain of autologous cell therapies. Cytotherapy 21(10):1081-1093. https://doi.org/10.1016/j.jcyt.2019.07.002.
- National Academies of Sciences, Engineering, and Medicine. 2020. Exploring Novel Clinical Trial Designs for Gene-Based Therapies: Proceedings of a Workshop. The National Academies Press, Washington, DC. http://doi.org/10.17226/25712.
- Roberts, M. 2020. Oxford/AstraZeneca Covid vaccine “dose error” explained. BBC. Available at: https://www.bbc.com/news/health-55086927 (accessed April 13, 2021).
- Rivière, I., & K. Roy. 2017. Perspectives on manufacturing of high-quality cell therapies. Molecular Therapy 25(5):1067-1068. https://doi.org/10.1016/j.ymthe.2017.04.010.
- Office of the Commissioner. 2021. FDA Takes Steps to Provide Clarity on Developing New Drug Products in the Age of Individualized Medicine. Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-steps-provide-clarity-developing-new-drug-products-age-individualized-medicine (accessed April 13, 2021).
- Callaway, E. 2020. Will the pandemic permanently alter scientific publishing? Nature 582:167-168. https://doi.org/10.1038/d41586-020-01520-4.
- National Academies of Sciences, Engineering, and Medicine. 2017. Building a National Capability to Monitor and Assess Medical Countermeasure Use During a Public Health Emergency: Going Beyond the Last Mile: Proceedings of a Workshop. The National Academies Press, Washington, DC. https://doi.org/10.17226/24912.
- Mervis, J. 2021. Biden’s First Budget Request Goes Big on Science. Science, April 9. Available at: https://www.sciencemag.org/news/2021/04/biden-s-first-budget-request-goes-big-science (accessed April 13, 2021).
- National Academies of Sciences, Engineering, and Medicine. 2021. Applying Systems Thinking to Regenerative Medicine: Proceedings of a Workshop. Washington, DC: The National Academies Press. https://doi.org/10.17226/26025.