Innovative Health Care Delivery: The Scientific and Regulatory Challenges in Designing mHealth Interventions
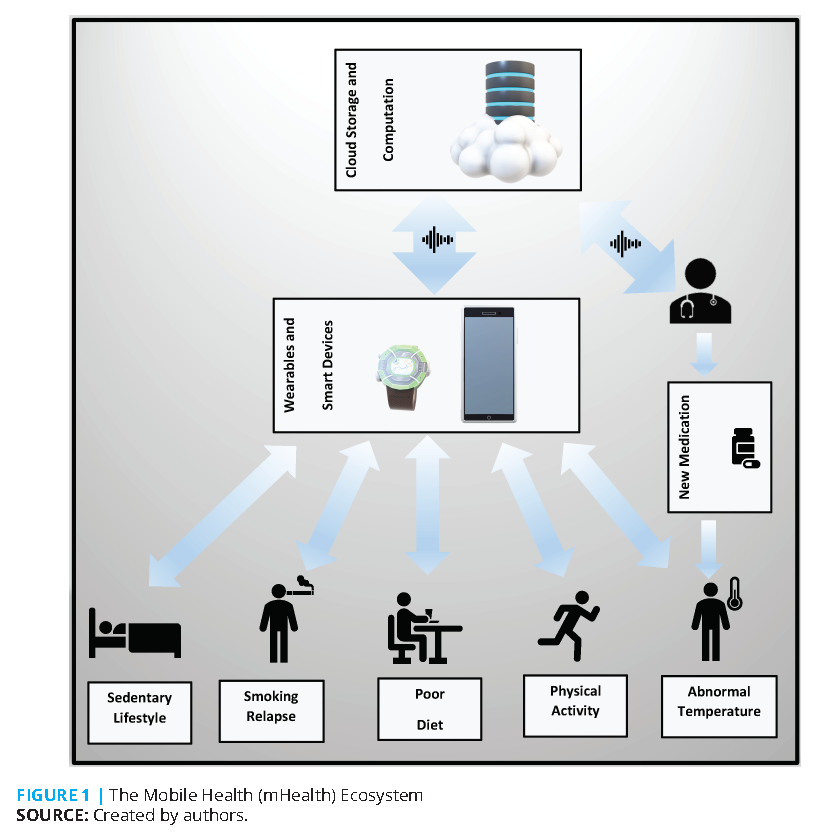
Scientists looking for innovative ways to deliver healthcare have long searched for mechanisms that can enable the right intervention to be delivered at the right time. Traditional delivery mechanisms have been limited both to the availability of a provider (e.g., a physician) and the location of care (e.g., a hospital or outpatient clinic). In recent years, however, numerous technological advancements—including wearable devices, mobile technologies, and the widespread development and use of user-friendly smartphone applications—have resulted in significant changes in how care is delivered. For example, mobile Health (mHealth) technologies are now commonly used to deliver interventions in a self-service and personalized manner, reducing the demands on providers and lifting limitations on the locations in which care can be delivered. Successful examples of mHealth interventions include programs to:
- maintain adherence to HIV medication and to smoking cessation efforts, which have shown sufficient effectiveness for adoption by health services [1];
- assist caregivers in managing veteran post-traumatic stress disorder (PTSD) and provide support with health care-related tasks within the Veterans Affairs (VA) system [2];
- continuously monitor chronic medical conditions, collect and share relevant data, and use this data to develop more effective treatment or disease management plans [1,3];
- encourage physical activity and weight loss in a more cost-effective, scalable manner than one-to-one approaches [1,4]; and
- reduce excessive alcohol use [1,4].
In addition, mHealth technologies enable governments and policymakers to more effectively respond to broader health care challenges, such as COVID-19 and future epidemics [5]. Notably, COVID-19 digital contact tracing apps (e.g., “Bluetooth-based exposure apps that trace proximity to other devices and GPS-based apps that collect geolocation data” [6]) have been widely used worldwide. For example, the number of active users of such apps in 2020 in Ireland, Switzerland, and Germany was reported to be about 1.3 million, 1.8 million, and 16 million, respectively [6].
These benefits of mHealth technologies have led numerous countries to roll out nationwide mHealth platforms or begin the process of doing so. National-level adoptions have been facilitated by WHO-led initiatives such as the mHealth Technical and Evidence Review Group (mTERG), the eHealth Technical Advisory Group (eTAG) [7], and the International Telecommunications Union-WHO Mobile Health for Non-Communicable Diseases Initiative, as well as by low-cost country-level health information systems such as District Health Information Systems (DHIS2) and Open Medical Record Systems (OpenMRS) [6,7]. The low cost of these systems has, in particular, allowed them to be easily and effectively used in low-income countries. For example, Mozambique started utilizing digital health nationwide in 2018 [8].
Given the rapid growth and wide use of these technologies, mHealth presents a number of urgent scientific and regulatory challenges for scientists, technology developers, policymakers, lawmakers, and other authorities. As the medical, government, financial, and technology sectors increasingly endorse the idea that mHealth can transform medicine [9], it is imperative that these challenges are addressed, as any effort to transform medicine through mHealth without doing so will likely be fraught with problems and ultimately unsuccessful.
In this light, the authors of this paper next discuss the main scientific and regulatory challenges facing the mHealth sector, and highlight the importance of addressing them to ensure that mHealth tools reach their full potential.
Scientific Challenges
Four sets of scientific challenges have impeded progress in the use of mHealth technology. First is the question of how to keep users engaged at the right level—neither under-engaged nor over-burdened—when deciding how and when to deliver treatment (e.g., sending a reminder to practice a stress regulation exercise). Developing best practices in confronting this challenge requires an understanding of the burden and the habitual consequences of how mHealth treatment encourages or forces engagement with users.
For example, if interventions are excessive, users may become disengaged, making interventions no longer effective. This is most pronounced in “push-based” mHealth interventions that interrupt the user throughout the course of the day, especially as mHealth interventions are increasingly becoming multi-component. As an example, in a multi-component push-based physical activity application, one component might send notifications to disrupt sedentary behavior while a weekly notification reminds and provides support for the individual to plan a fun physical activity for the subsequent week. Extensive deliveries by one component—for example, too many reminders about sedentary habits—can reduce the effectiveness of the other component, either by increasing user burden or resulting in habituation, both of which can eventually lead to disengagement. Disengagement, in turn, can reduce the quality or the quantity of the data needed by scientists to find ways to design more effective interventions. As a result, scientists need to gain an understanding of the complex interplay among components and ensure that mHealth interventions are designed in such a way to minimize the likelihood of overburden or habituation. In “pull-based” interventions, such as graphs of physical activity available 24-7 for self-monitoring, concerns of increased burden or habituation are not as prominent. However, in such interventions, scientists still need to address a similar concern: ensuring that users remain effectively engaged and interested in participating over time.
The second scientific challenge is how to tailor the content of an mHealth treatment as well as its delivery to a user’s current context. In using mHealth technologies for interventions known to be effective when delivered in other ways (e.g., person-to-person), scientists will likely need to adapt the intervention to take advantage of the new delivery mode, as well as to accommodate the constraints imposed by smart device screens or speakers. In addition, new interventions are increasingly being developed specifically for delivery through mHealth technologies. For both known and new interventions, scientists need to understand how to adapt the content of the treatment as well as its delivery on the user’s current context (e.g., mood, location, weather, etc.). Here, innovative post-intervention experimental research on time-varying mediators and moderators, including unobservable factors (e.g., the emotional state of the user), are often needed to provide data that can be used to make interventions more effective. Insight for improving the delivery of mHealth services can also be gleaned from current experimental design methods used to optimize large-scale systems, many of which are already being used in various industries (e.g., the adaptive optimization of advertisements by Google, or the optimization of routes and assignment of drivers to incoming ride-sharing requests by Uber).
The Micro-Randomized Trial (MRT) is a variant of traditional experimental design that can be used to provide the data needed to understand how best to adapt the content and delivery timing of a treatment to the user’s current context. In classical MRTs, treatment is repeatedly randomly assigned at multiple time points for each user (e.g., time points when a treatment is potentially effective based on theory, the user’s past behavior, or the user’s current context) [10]. Classical MRTs rely solely on “offline learning,” which means that the data are analyzed for treatment interactions, moderation by context, and other variables only after the study is over. A drawback is that the study participants do not necessarily derive any benefit from these learnings during the study itself. Thus, scientists often need to make use of new experimental designs that facilitate continuous, within-user optimization of treatment content or delivery.
One option for an improved MRT design is to allow the randomization probabilities to be adaptively changed in favor of better-performing treatments. This can be achieved via use of “online learning” approaches from artificial intelligence (AI)—in particular, the AI method of reinforcement learning—that allow treatment delivery to be continually optimized to the user’s current context [11]. Notably, AI approaches can also enable online learning of effective predictors of negative outcomes (e.g., relapse to smoking) for each individual and can keep track of changes in these predictors (as measures of the user’s current context), triggering suitable preventive interventions when needed.
The third challenge in developing mHealth interventions is that they should preferably abide by the well-known principle of “primum non nocere” (“first, do no harm”). Achieving this is particularly difficult given that the interventions are not delivered face to face. To begin with, prompting users during inappropriate times or emotional states may result in serious harms. For example, prompting a user for engagement while they are driving can lead to an accident, and prompting a user to stop smoking while they are in an unsuitable emotional state may increase the individual’s stress level, resulting in more smoking. Therefore, scientists need to include suitable detection mechanisms that can be used in real-time to avoid such harms. Further, if an mHealth intervention provides social support by connecting a user to other users or to an online support group, the inter-user exchanges must be appropriately managed to prevent disruptive, harmful messages. These and other related ethical issues are of high importance, and paying attention to them should be a primary part of the scientists’ design plan.
Finally, mHealth interventions need to be designed to balance objectives on proximal and distal health outcomes. Understanding the causal chain linking the proximal effects of the treatments to longer-term health outcomes can significantly improve mHealth delivery. However, it is often difficult to measure both proximal and distal outcomes, and furthermore, it is a perplexing task to understand the linkages between the two. For example, in physical activity applications, interventions are supposed to contribute, in the long term, to the formation and maintenance of stable, positive activity habits. While mHealth interventions might target proximal outcomes such as the number of steps taken during the course of a day, scientists need to understand how gains in this area might lead to the formation of longer-term physical activity habits.
Regulatory Challenges
The increased use of mHealth technologies has also created new regulatory challenges for policymakers, lawmakers, and other authorities. Most notable is the importance of protecting user data. Advances in data fusion technologies have enabled devices and sensors connected via the “Internet of Things” to collect data from (and communicate with) each other [12]. While this has resulted in designing and delivering more effective interventions, it has simultaneously increased the risk of data breach and misuse. Thus, new regulatory avenues are required to ensure that the mass adoption of such technologies does not compromise data privacy, especially in interventions that make use of Electronic Medical Records (EMRs). At the same time, these regulatory avenues should not be so restrictive as to discourage data fusion or related data sharing activities (e.g., when a patient tries to use multiple mHealth applications, each of which might be working with a different EMR system) that can allow scientists to design superior mHealth interventions.
Considering these challenges, the Food and Drug Administration (FDA) in a 2019 statement encouraged “the development of mobile medical apps (MMAs) that improve health care” but also emphasized its “public health responsibility to oversee the safety and effectiveness of medical devices—including mobile medical apps” [13]. The FDA’s MMA guidance, first issued in 2013, was updated in 2019 “to reflect changes to the device definition in accordance with Section 3060 of the 21st Century Cures Act, which created a function-specific definition for device” [13]. There remains, however, a great deal of ambiguity in the FDA regulatory definition of “device.” Furthermore, for many mHealth applications, the FDA exercises enforcement discretion [13] and does not oversee the development or use of the application. The FDA’s overall oversight of mHealth technologies has been controversial among members of Congress and industry [14], and has been also criticized by academics [9].
Another legal concern centers on the consequences of failing to respond in a timely manner to mHealth technology alerts—particularly those used to continuously monitor chronic conditions and to implement disease management plans [15]. This is especially the case for caregivers (e.g., medical staff or family members), who might be already overburdened with other duties. Adverse events caused by such oversight can create various legal ambiguities; for example, which entity is responsible, and are such events covered by insurance plans?
Conclusion and Looking Forward
The use of mHealth interventions has been rapidly increasing worldwide as health care providers, industry, and governments seek more efficient ways of delivering health care. Despite the technological advances, increasingly widespread adoption, and endorsements from leading voices from the medical, government, financial, and technology sectors, multiple key challenges remain unaddressed. Addressing these challenges requires urgent attention as well as close collaboration among scientists, policymakers, lawmakers, and other authorities. Without such attention and collaboration, the real value of mHealth technologies will be left significantly untapped.
Join the conversation!
![]() Tweet this! Mobile health tech allows clinicians to reach patients outside of the hospital/clinic and offer promises of tailored and individualized care. A new #NAMPerspectives outlines steps that could be taken to optimize the use of these technologies: https://doi.org/10.31478/202108b
Tweet this! Mobile health tech allows clinicians to reach patients outside of the hospital/clinic and offer promises of tailored and individualized care. A new #NAMPerspectives outlines steps that could be taken to optimize the use of these technologies: https://doi.org/10.31478/202108b
![]() Tweet this! Micro-randomized trials should be considered as a method for collecting data necessary to adapt and tailor mobile health technologies to serve individual patients in the context and mental state they are in at the moment. Read more: https://doi.org/10.31478/202108b
Tweet this! Micro-randomized trials should be considered as a method for collecting data necessary to adapt and tailor mobile health technologies to serve individual patients in the context and mental state they are in at the moment. Read more: https://doi.org/10.31478/202108b
Download the graphics below and share them on social media!
References
- Free, C., G. Phillips, L. Galli, L. Watson, L. Felix, P. Edwards, P. Patel and A. Haines. 2013. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Medicine 10(1):1–45. https://doi.org/ 10.1371/journal.pmed.1001362.
- Frisbee, K. L. 2016. Variations in the Use of mHealth Tools: The VA Mobile Health Study. JMIR Mhealth Uhealth 4(3):e89. https://doi.org/10.2196/mhealth.3726.
- Nilsen, W., S. Kumar, A. Shar, C. Varoquiers, T. Wiley, W. T. Riley, M. Pavel, and A. A. Atienza. 2012. Advancing the Science of mHealth. Journal of Health Communication 17 Suppl 1:5-10. https://doi.org/10.1080/10810730.2012.677394.
- Afshin, A., D. Babalola, M. McLean, Z. Yu, W. Ma, C-Y. Chen, M. Arabi, and D. Mozaffarian. 2016. Information Technology and Lifestyle: A Systematic Evaluation of Internet and Mobile Interventions for Improving Diet, Physical Activity, Obesity, Tobacco, and Alcohol Use. Journal of the American Heart Association 5(9):pii: e003058. https://doi.org/10.1161/JAHA.115.003058.
- Adans-Dester, C. P., S. Bamberg, F. P. Bertacchi, B. Caulfield, K. Chappie, D. Demarchi, M. K. Erb, J. Estrada, E. E. Fabara, M. Freni, K. E. Friedl, R. Ghaffari, G. Gill, M. S. Greenberg, R. W. Hoyt, E. Jovanov, C. M. Kanzler, D. Katabi, M. Kernan, C. Kigin, S. I. Lee, S. Leonhardt, N. H. Lovell, J. Mantilla, T. H. McCoy, N. M. Luo, G. A. Miller, J. Moore, D. O’Keeffe, J. Palmer, F. Parisi, S. Patel, J. Po, B. L. Pugliese, T. Quaitieri, T. Rahman, N. Ramasarma, J. A. Rogers, G. U. Ruiz-Esparza, S. Sapienza, G. Schiurring, L. Schwamm, H. Shafiee, S. Kelly Silacci, N. M. Sims, T. Talkar, W. J. Tharion, J. A. Toombs, C. Usching, G. P. Vergara-Diaz, P. Wacnik, M. D. Wang, J. Welch, L. Williamson, R. Zafonte, A. Zai, Y-T. Zhang, G. J. Tearney, R. Ahmad, D. Walt, and P. R. Bonato. 2020. Can mHealth Technology Help Mitigate the Effects of the COVID-19 Pandemic? IEEE Open Journal of Engineering in Medicine and Biology (1):243-248. https://hdl.handle.net/1721.1/128713.
- Blasimme, A., and E. Vayena. 2020. What’s Next for COVID-19 Apps? Governance and Oversight. Science 370(6518):760-762. https://doi.org/10.1126/science.abd9006.
- Mehl, G., and A. Labrique. 2014. Prioritizing Integrated mHealth Strategies for Universal Health Coverage. Science 345(6202):1284-1287. https://doi.org/10.1126/science.1258926.
- Pallares, G. 2018. Mozambique Is Rolling Out Its mHealth Platform Nationwide — and This is How. devex. Available at: https://www.devex.com/news/mozambique-is-rolling-out-its-mhealth-platform-nationwide-and-this-is-how-91913 (accessed July 7, 2021).
- Cortez, N. G., I. G. Cohen, and A. S. Kesselheim. 2014. FDA Regulation of Mobile Health Technologies. New England Journal of Medicine 371(4): 372-379. https://doi.org/10.1056/NEJMhle1403384.
- Klasnja, P., E. B. Hekler, S. Shiffman, A. Boruvka, D. Almirall, A. Tewari, and S. A. Murphy. 2016. Microrandomized Trials: An Experimental Design for Developing Just-In-Time Adaptive Interventions. Health Psychology 34S:1220-1228. https://doi.org/10.1037/hea0000305.
- Liao, P., K. Greenewald, P. Klasnja, and S. A. Murphy. 2020. Personalized HeartSteps: A Reinforcement Learning Algorithm for Optimizing Physical Activity. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 4(1):1-22. https://doi.org/10.1145/3381007.
- Saghafian, S., B. T. Tomlin, and S. Biller. The Internet of Things and Information Fusion: Who Talks to Who? Manufacturing and Service Operations Management (M&SOM), forthcoming.
- U.S. Food and Drug Administration. 2019. Device Software Functions Including Mobile Medical Applications. Available at: https://www.fda.gov/medical-devices/digital-health-center-excellence/device-software-functions-including-mobile-medical-applications (accessed July 7, 2021).
- House Committee on Small Business, Subcommittee on Health and Technology. 2013. Mobile Medical App Entrepreneurs: Changing the Face of Health Care. Available at: http://www.gpo.gov/fdsys/pkg/CHRG-113hhrg81702/pdf/CHRG-113hhrg81702.pdf (accessed July 7, 2021).
- Edwards-Stewart, A., C. Alexander, C. M. Armstrong, T. Hoyt, and W. W. O’Donohue. 2019. Mobile Applications for Client Use: Ethical and Legal Considerations. Psychological Services 16(2): 281–285. https://doi.org/10.1037/ser0000321.