Individual Patient-Level Data Sharing for Continuous Learning: A Strategy for Trial Data Sharing

The National Academy of Medicine (NAM) has prioritized the use of clinical and administrative health care data as a core utility for a continuously learning health system (The “Learning Health System” is a system in which science, informatics, incentives, and culture are aligned for continuous improvement and innovation, with best practices seamlessly embedded in the care process, patients and families as active participants in all elements, and new knowledge captured as an integral by-product of the care experience. SOURCE: Institute of Medicine. 2013. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press) and for advancing the health and health care of Americans. There is increasing acceptance that sharing data constitutes a key strategy for continuous and real-time improvement in the effectiveness and efficiency of patient care and for the enhancement of research transparency and reproducibility [1]. “Individual patient-level data (IPD) (This includes individual patient-level data (e.g., raw data or an analyzable dataset); metadata, or “data about the data” (e.g., protocol, statistical analysis plan, and analytic code); and summary-level data (e.g., summary-level results posted on registries, lay summaries, publications, and clinical study reports)) sharing” refers to “widespread, third-party access to the IPD and associated documentation from clinical trials” to achieve broad societal and scientific benefits [2].
Analyses of existing IPD may lead to a better understanding of current evidence, the generation of new information to support informed health care decision making, and improved transparency of original research findings, which, in turn, may enhance data integrity and public confidence in the overall clinical trial enterprise [3,4,5]. Public registration of key study details at study inception and the reporting of summary results through platforms such as ClinicalTrials.gov and other registries in the World Health Organization International Clinical Trials Registry Platform have already improved clinical research transparency. IPD sharing represents the next step in facilitating the transformation of raw study data to the aggregated data that form the basis of statistical analyses and reported results [2]. Making IPD and associated metadata available after study completion for clinical trials and observational studies can benefit the research community by enhancing transparency and enabling careful examination of the data and methods used by the primary research team (e.g., as demonstrated in Box 3), which is important, given ongoing concerns about the reproducibility of research studies [6]. Although industry has different incentives and concerns from academia, for academic investigators, the benefits of data sharing include preservation and accessibility of their data, increased citation of the work, and increased visibility and opportunity for new collaborations [7].
Although there are potential benefits to IPD sharing, there are also many barriers that have yet to be addressed [6,8,9,10,11]. Contentious issues include consent for data sharing and the sharing of anonymized data, sustainable infrastructure and resources to support the preparation of IPD and metadata, and the heterogeneity of data repositories and related tools [9,12,13]. Also, limited guidance exists on the role of the primary research team in preparing IPD, the responsibilities of secondary research teams to ensure valid analyses, and the process by which conflicting findings should be reconciled [14,15]. For example, Natale, Stagg, and Zhang, and Gay, Baldridge, and Huff man demonstrate the potential difficulty of IPD reanalysis, given differences in population and endpoint definitions, and reliance on primary investigators to explain datasets and facilitate data use [16,17].
While not focused exclusively on sharing IPD, the Future of Research Communications and e-Scholarship group (FORCE11), as a step to address some of these barriers, published the first iteration of the FAIR (findable, accessible, interoperable, and reusable) principles in 2016, which aim to improve management and stewardship [18]. More recently, the International Committee of Medical Journal Editors (ICMJE) issued a statement on data sharing for clinical trials. As of July 2018, all ICMJE member journals began requiring that articles reporting results from clinical trials include a data-sharing statement. (According to the ICMJE website, data-sharing statements must include the following information: “whether individual deidentified participant data (including data dictionaries) will be shared (‘undecided’ is not an acceptable answer); what data in particular will be shared; whether additional, related
documents will be available (e.g., study protocol, statistical analysis plan, etc.); when the data will become available and for how long; by what access criteria data will be shared (including with whom, for what types of analyses, and by what mechanism).” SOURCE: International Committee of Medical Journal Editors. 2019. Recommendations: Clinical trials. http://www.icmje.org/recommendations/browse/publishingand-editorial-issues/clinical-trial-registration.html#two (accessed May 15, 2019).) Furthermore, for any clinical trials that began enrolling participants after January 1, 2019, the data-sharing plan needs to be included as part of the trial’s registration [19].
Additionally, articles by Ohmann and colleagues offer consensus-based principles and recommendations for addressing common barriers, such as incentivizing, resourcing, and planning for IPD sharing during the design of an original study; structuring data and metadata using widely recognized standards; managing repository data and access; and monitoring data sharing [9,12]. Finally, the responsible sharing of clinical trial data was also the focus of a 2015 Institute of Medicine (now NAM) report, Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk, that offers guiding principles for responsible data sharing and describes the benefits, risks, and challenges for a variety of stakeholders, including participants, sponsors, regulators, investigators, research institutions, journals, and professional societies [8].
The present paper aims to describe strategies for addressing outstanding challenges to IPD sharing that were identified through a collaborative effort facilitated by the NAM and through a review of relevant literature and selected IPD repositories. It builds on previous efforts by providing specific case examples of IPD sharing efforts (several of which are being led by members of the author group), focusing specifically on issues of greatest relevance to the US context and considering data from both commercial and non-commercial sources. While the authors present multiple viewpoints on how best to share IPD, all believe that important scientific contributions may be derived from leveraging previously acquired data for additional research and analysis.
The NAM Collaboration
To discuss the outstanding questions related to IPD sharing, the NAM hosted a meeting of the Clinical Effectiveness Research Innovation Collaborative in November 2016. During the discussion, meeting participants called for a more substantial and strategic focus on how to facilitate IPD sharing effectively, efficiently, and ethically. To address this charge, the authors of this paper have collected examples and drawn from their personal experiences to develop a set of actionable steps that may help promote responsible and widespread sharing of IPD from clinical trials that involve participants from the United States. We also identify the stakeholders responsible for each of the identified action steps.
Our paper does not describe all possible benefits and harms that may be associated with IPD sharing initiatives, nor does it include all possible financial considerations. Instead, the purpose is to create a policy and practice agenda that could lead to more robust and evidence-based IPD sharing efforts within the United States. Enhancing continuous learning from further analyses and the study of original data, without additional risk to patients and with maximum benefit for society, requires work, resources, culture change, and collaboration. To promote the necessary changes, we focus on operationalizing Recommendations 1, 3, and 4 of the NAM Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk publication: developing a data-sharing culture; implementing operational strategies to maximize benefits and minimize risks; and addressing infrastructure, technological, sustainability, and workforce challenges associated with IPD sharing [8].
Examples of IPD Sharing Initiatives
Over the past decade, there has been meaningful progress in activities, regulation, and practices associated with IPD sharing. Several governmental and nongovernmental agencies have either developed or are in the process of developing guidance related to IPD sharing, clinical study reports, summary results, and trial registration (e.g., the European Medicines Agency [EMA], ICMJE, the National Institutes of Health [NIH], the US Department of Veterans Affairs, the US Food and Drug Administration [FDA], and the World Health Organization) [19,20,21,22,23,24,25,26,27,28]. Broader policy changes have also emerged. For example, the European Union’s General Data Protection Regulation regarding individual data privacy and accountability may have consequences for clinical research and patient care [29]. Many pharmaceutical companies have also established their own policies for IPD sharing [30], and
data sharing is encouraged and incentivized through various federal regulatory and funding agencies and publication bodies. While the requirements of these policies do not always directly align, they are important steps in IPD data sharing.
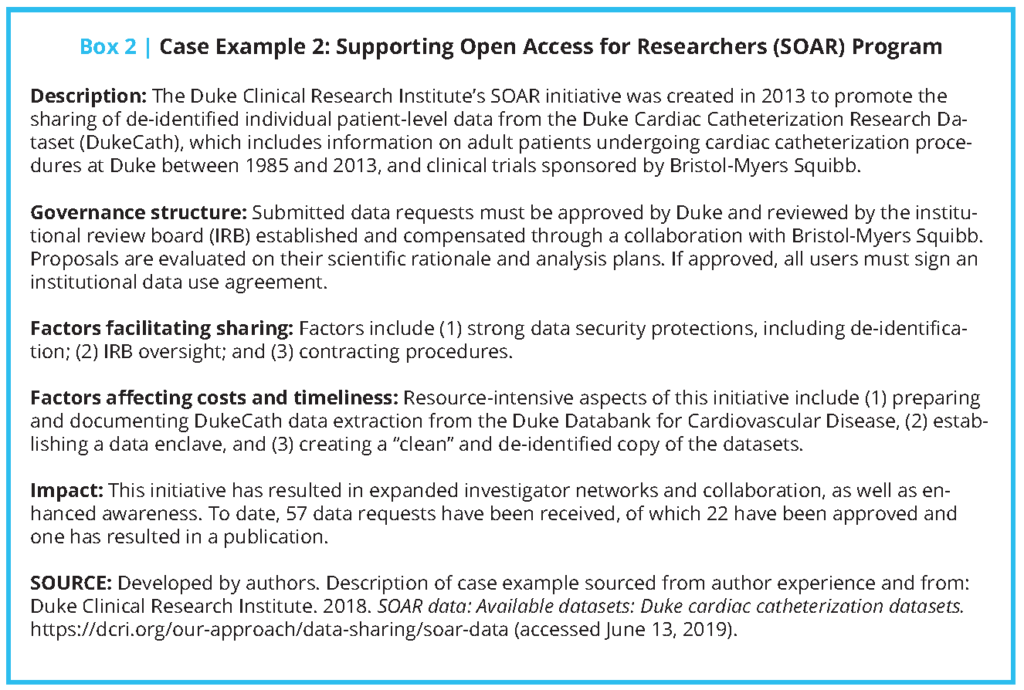
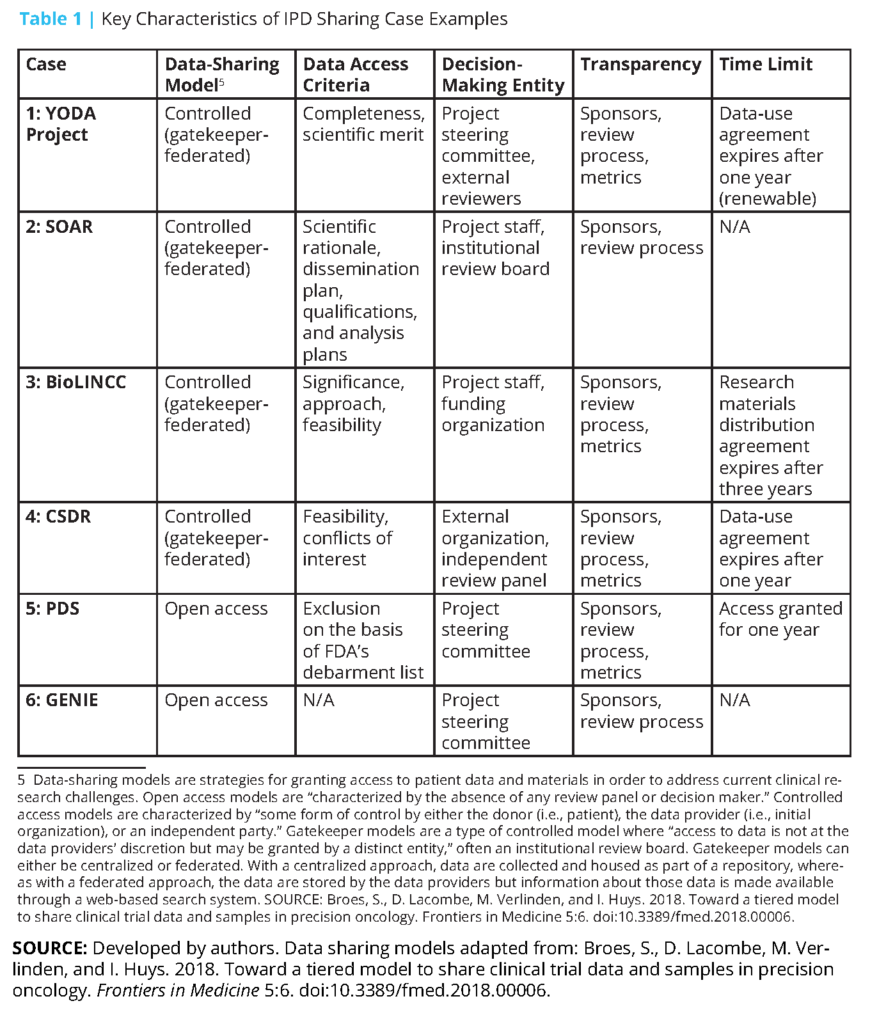
Additionally, several public funders and private companies—particularly some pharmaceutical and medical device companies that sponsor and conduct clinical trials—have established data repositories for secondary use and analysis [31,32,33,34,35,36,37]. Since effective clinical trial IPD sharing requires maintenance of and adequate support for data repositories, we examined six case studies of established repositories that have navigated obstacles to creating an infrastructure, developed operational strategies to maximize benefit and minimize risk, and contributed to growing a data-sharing culture. (These examples are not meant to represent an exhaustive list of available data repositories. Other repositories not discussed, but that may be of interest, include Vivli (https://vivli.org/), OpenTrials (https://opentrials.net/), and Dryad (https://datadryad.org).) We described each system’s governance structure, factors facilitating sharing, factors affecting cost and timeliness, and impact to date (see Boxes 1-6 and Table 1). The specific cases included are the Yale University Open Data Access (YODA) Project (Case 1); Duke Clinical Research Institute’s Supporting Open Access for Researchers (SOAR) (Case 2); the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) of the National Heart, Lung, and Blood Institute (NHLBI) (Case 3); ClinicalStudyDataRequest. com (CSDR) (Case 4); Project Data Sphere (PDS) (Case 5); and the Project Genomics Evidence Neoplasia Information Exchange (GENIE) of the American Association for Cancer Research (AACR) (Case 6).
Of these six cases, a few were included because of the authors’ detailed knowledge regarding these efforts, and others were added because of the availability of information on their development and procedures. The six cases were also selected because they differ in several important ways related to governance, data access models, data availability, and data type. For example, some are administered by public sector organizations and include data from publicly funded studies, whereas others are administered by groups of private sector organizations or public-private partnerships and include data from industry studies. Some use open-access data-sharing models, in which there is minimal review of data requests, while others rely on controlled access models, which use in-house or third-party expert review of data requests to provide greater protection for patients and data sponsors [38,39]. Some offer data contributors the opportunity to review data requests for potential conflicts in terms of their publication plans, whereas others offer data users broad access to all available data.
The approaches these repositories take to collecting and accessing IPD vary. AACR’s GENIE consists of voluntarily contributed data that are required to meet criteria related to quality and comprehensiveness, and must include at least 500 genomic records. The YODA Project and SOAR largely rely on robust partnerships among a small group of academic and industry data holders. Across repositories, IPD are required to be de-identified in a manner that is consistent with the Health Insurance Portability and Accountability Act and shared in a manner that is consistent with participants’ informed consent in cases in which the data were not de-identified.
For example, NHLBI’s BioLINCC repository, which includes data from NHLBI-funded studies, requires those contributing IPD to specify whether their data were collected with broad, unrestricted consent or tiered consent, and limits secondary uses in a manner that is consistent with consent restrictions (e.g., data can only be used for research on certain topics). BioLINCC also requires that data from funded contracts and large grants be made available within two years after the publication of primary outcome data, with additional rules for observational studies [40]. BioLINCC’s historical development, described by Giff en et al. and Coady and Wagner [40,41], demonstrates the complex decisions underlying IPD repositories. BioLINCC’s development entailed organizing existing data; assessing its quality; developing documentation for preparing, submitting, and requesting datasets; and developing workflows for data requests and review processes [40,41].
Coady et al. describe the use and publication record of BioLINCC. From January 2000 to May 2016, the repository received 1,116 data requests for 100 clinical studies [32]. Five years after the data request, 35 percent of studies that reused clinical trial data and 48 percent of studies that reused observational data were published [32]. A survey of investigators who had received data from BioLINCC indicated that due to time or financial resources, it was not practical to collect data of similar size and scope as those originally collected via NIH-supported work and made available through BioLINCC [42].
An analysis of data reuse requests to the YODA Project, SOAR, and CSDR indicated that between 2013 and 2015, 234 proposals were submitted [43]. For the YODA project, the vast majority of investigators requesting data (91.5 percent) are from academic institutions [44]. Although data request and data use statistics are useful indicators of IPD sharing, additional data are needed to better understand how instances of IPD sharing directly enhance patient care and quality improvement. Studies of data requests from BioLINCC suggest that investigators use data for novel research questions (72 percent), meta-analyses (7 percent), or pilot studies (9 percent); relatively few requested data for reanalysis [30,40]. These studies have also demonstrated that BioLINCC is most used by early stage investigators or trainees, which suggests “a potential role for repositories in the development of new trialists and epidemiologists” [32].
While growth in the use of clinical data repositories is promising, an important challenge for these repositories is the curation of the data, including data provenance, data formatting, and metadata quality [45]. The FDA has issued several guidance documents on data formats, metadata requirements, and related information, and since 2017, it has required that data be submitted in a format that adheres to the Clinical Data Interchange Standards Consortium (CDISC) standards [46,47]. Through Policy 0070, uniform data preparation and documentation will also soon be required for any medical product trial submitted to the EMA. The policy currently requires publication of clinical study reports but will include IPD at a later date [21]. Similarly, as noted in the case examples, data must be uniformly indexed or cataloged so that they can be located when requests are received. For BioLINCC, it took between 85 and 350 hours to prepare IPD and supporting materials for each individual study, depending on data complexity and documentation quality [32]. Given the costs and effort associated with data sharing, there is a need to deploy educational efforts that encourage researchers to use robust clinical trial designs and develop clear and usable metadata and documentation as part of trial conduct, and provide guidance on developing data-sharing efforts in collaboration with stakeholders. There is also a need to improve the efficiency of IPD preparation, incentivize publication, and support the costs of data curation.
IPD Sharing Opportunities and Obstacles
Based on our review of data repositories, as well as findings from NAM’s Clinical Effectiveness Research Innovation Collaborative and our individual experiences, we have identified five opportunities for addressing the critical obstacles to sharing IPD from clinical trials (see Box 7). In this section, we describe specific tasks for key stakeholders—including research teams, secondary data users, journal editors, research funders, data repository owners, institutional review boards, and others—to consider in order to take full advantage of the opportunities afforded by sharing clinical trial data. Similar discussions are occurring around data generated through longitudinal studies; routine health care delivery and health delivery system data warehouses; and patient-reported outcomes [48,49,50,51].
While there are unique considerations associated with all of these data resources, there are also several commonalities, many of which are reflected in the following discussion.
1. Improve Incentives for Data Sharing for Primary Researchers and Research Institutions, Including Academic Credit for the Generation of Rich Data Sources That Are Shared and Used
In most research fields, researchers are incentivized to maintain IPD ownership and not share with the wider scientific community, to maximize publication and other traditionally valued academic opportunities. To incentivize sharing within and among academic institutions, it may be necessary to develop a system of academic credit for trialists who generate and share data that acknowledges the effort required to conduct clinical trials and organize, clean, and store IPD; and that provides meaningful credit and other incentives for making that data available to others. Academic institutions should reward, celebrate, and highlight investigators who share, particularly those whose work leads to downstream contributions.
One of several recent suggestions is that publications identify the source and location of the datasets used as the basis of the manuscript—linking the researchers who make substantial contributions to data acquisition, quality control, creation and authoring of metadata, and curation—to assist in providing academic credit for these efforts [52,53]. The appropriate mechanism for making such attributions is a topic requiring continued discussion given the potentially large number of individuals involved in producing and curating health data [53]. Additionally, research journals should consider requesting specification of the data source as a citable reference to ensure that the researchers who collected the data and the data stewards receive credit in resulting publications and repositories should provide citations for each data set included, following the example set by Dryad.
Research funders should also consider strategies to promote IPD sharing. For example, funders could require IPD sharing prospectively in appropriate requests for proposals, providing clear instructions regarding data-sharing expectations; providing repositories and an integrated process, such as the one used by BioLINCC (see Box 3); and, as previously mentioned, including additional infrastructure support to help cover the costs of sharing, which include organizing and managing data for reuse, governance, and oversight.
An additional concern is that even after fulfilling sponsor agreements and other regulatory requirements for data sharing, without appropriate incentives for all of the parties involved, data could be shared without appropriate metadata or other needed context and resources. Further discussion is needed on developing a clearer understanding of jurisdiction, ownership, and responsibility for shared data and metadata (see topic five below). This exploration of the various forces that could help facilitate the process of IPD sharing and consideration of potential action items may promote necessary change in culture and practices in clinical research.
2. Create General Rules to Address Patient Consent and Privacy Issues, Anticipating Future Secondary Analyses and Sharing of Primary Clinical Trial Data
To minimize the risk and maximize the benefit of data sharing, primary research teams, institutional review boards, the federal offices of human research protection (the US Department of Health and Human Services Office for Human Research Protections, the US Department of Veterans Affairs Office of Research Oversight, and the US Department of Defense Human Research Protection Office), and associated institutions should work to address patient consent and privacy issues to anticipate future secondary analyses and sharing of primary clinical trial data. To achieve this, a general framework should be developed to determine whether consent for secondary data use is needed from participants at the beginning of a new study, how to communicate that data collected will be shared, how to exclude or contact individuals who do not want their data shared without explicit consent, how to ensure data are sufficiently de-identified for new analyses, and how to streamline the development and use of appropriate data-use agreements. Ohmann et al. suggest several practices for attaining consent for secondary use of data, including offering a lay explanation of the potential benefits and harms of data sharing; how the data will be prepared, stored, and accessed; and the practical difficulty of trial participants withdrawing their consent [9].
In addition, where possible, data intended to be shared should be prepared with de-identification in mind. Both PLoS and ICMJE data policy indicate that investigators should share de-identified data underlying their published clinical trials results [19,54]. Additional guidance regarding novel and standardized strategies to de-identify data should be broadly disseminated. In particular, there is a need for a conceptual framework and terminology describing potential causes and consequences of re-identification and potential types of identifiers and their risk of re-identification. (A preliminary overview of these concepts is provided in the Institute of Medicine’s 2015 report Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk (https://doi.org/10.17226/18998).) Members of the public and study participants should be engaged in the development of such a framework. The potential risks of re-identification, which may increase if de-identified IPD are combined with existing public information, include violation of patient privacy and medical identity theft, and may disproportionately affect minorities [15]. Repositories such as the YODA Project (see Box 1) include language in their data use agreements that explicitly forbids activities that may cause re-identification.
3. Consider the Operational Expenses Associated with Data Repositories and Develop a Framework to Identify the Stakeholders and Resources Necessary to Cover Those Operational Costs
According to Wilhelm, Oster, and Shoulson: “The investigators who lead the Alzheimer’s Disease Neuroimaging Initiative have estimated that across the lifetime of the nearly $130 million project, 10% to 15% of the total costs will have been dedicated to data-sharing activities and that investigators will have spent about 15% of their time on data-sharing tasks, such as uploading data or responding to queries from outside researchers” [55]. While this example is illustrative, it is important to recognize that costs can vary substantially based on several factors, including the model used for data sharing and access, availability of technical assistance, the extensiveness of procedures for reviewing data requests, and the need and intensity for legal review of DUAs. If IPD sharing is to be more widely adopted, the associated costs should be better understood by investigators, their institutions, repositories, and research funding organizations, and such costs should be potentially included as part of the research process. There are also recurring costs associated with data curation and data repositories that need a reliable funding stream. For example, to ensure that appropriate personnel are available to review reanalysis requests, it is necessary to have sufficient funds to support staff time after the normal conclusion of a study. These costs may also be borne by repositories such as CSDR (see Box 4), which relies on independent external reviewers, and PDS (see Box 5), which allows data sponsors to conduct an additional review of risks related to intellectual property and competitive advantage. Additionally, data repositories require considerable resources to provide governance, such as the development of common data request forms and processes.
A list of potential data-sharing tasks that may require funding, based on costs highlighted by case examples, is provided in Box 8. While it may be possible for primary researchers and repository owners to write some of these additional costs into funding requests, another possibility might be for funding agencies to consider separate funding streams for IPD sharing, since many funders may have an interest in extending the impact of their grantmaking by making data from funded studies easier to share. Funders could also consider developing mechanisms that support secondary research projects conducted either by the original research team or by other investigators. Ohmann et al. suggest avoiding access fees to data where possible, but they note that in some cases, “the costs of preparing data for sharing may need to be met by the secondary users” [9]. While promoting access by avoiding user fees may be possible in some situations, data repositories should be able to develop their own business models based on their needs and existing support. Existing secondary research using administrative claims data, such as Medicare and Truven Health MarketScan data, may offer useful contracting models and data-use agreements.
4. Develop a Conceptual Framework to Specify What Clinical Trial Data and Associated Metadata Should Be Shared, Organized, and Stored, Including Whether Stored Data Should Be Raw or Derived
To achieve widespread sharing of IPD, it is necessary to establish a framework and standards for data documentation, organization, and storage, for processes related to coding and analysis, and for ensuring appropriate personnel are available to review reanalysis requests and expedite decision making. Collected data should use standardized formats that facilitate use by secondary research teams and merge data from multiple trials and sponsors where possible. In the absence of such standards, primary investigators and others may be burdened by having to develop post hoc informatics tools to transform data in order to facilitate use [53]. For example, although the CDISC is widely used by industry because the data it contains follow formats required by the FDA and EMA, it is not used by the NIH and its funded researchers, which do not have such format requirements.
In addition to data standardization, it is necessary for trialists to share key metadata, including protocols, data dictionaries, statistical analysis plans, and template case report forms. To facilitate more consistent metadata across repositories, Canham and Ohmann propose a schema for metadata that captures, “(a) study identification data, including links to clinical trial registries; (b) data object characteristics and identifiers; and (c) data covering location, ownership and access to the data object.” [568 In addition, repositories such as the YODA Project (see Box 1) require sponsors to prepare metadata and other documentation for external sharing to allow for subgroup analyses and other uses. With respect to clinical trial protocols, several of the repositories described in the case examples, such as PDS (see Box 4), include study protocols in their data query system, while others—such as the YODA Project (see
Box 1) and CSDR (see Box 3)—provide access to protocols upon approval of data requests. Ohmann et al. provide suggestions on how to develop consistent citable identifiers for repositories, protocols, and datasets [9]. One potential action item for improving metadata is building on existing literature to develop standards or guidelines for investigators to facilitate defining derived variables, provide the rationales for defining such derived variables, and describe the expected responsibilities of secondary research teams aiming to perform replication or subgroup studies [57. Similar guidance has been developed for secondary analyses of administrative and health care data and may be used as a model [58,59].
5. Develop Guidelines For How Data Repositories Can Promote Meaningful Data Sharing, and for How to Select an Appropriate Repository Platform
Repository platforms provide data holders with the ability to share their data in a systematic and accessible way. As described in Table 1, data repositories rely on a range of data access models, review criteria and bodies, data-use agreements, and data-sharing and analysis platforms. Decisions regarding these factors should be made based on their alignment with the type and scale of data being stored. For example, GENIE relies on cBioPortal, an open source platform that is uniquely engineered to support analyses and visualizations of cancer genomics data. Additionally, repository owners should provide appropriate data security for data transfer and analysis systems and overall governance, such as the development of common data request forms and processes. Instructions on how to use repositories’ analysis environments should be made available to researchers. Repositories should engage in discussion and planning to determine how data repositories should interoperate to reduce the potential problems associated with having different datasets available in varied data-sharing platforms and repositories with
different requirements.
Conclusion
Sharing of IPD from clinical trials and, eventually, widespread sharing of routinely generated electronic health information is necessary for realizing the vision of a continuously learning health system. The obstacles and opportunities described in this paper are meant to contextualize actionable steps that should be taken by key stakeholders to engage in IPD sharing. We realize that these obstacles and opportunities will continuously evolve with the increase of IPD sharing initiatives and new lessons learned. In that vein, we would encourage pilot testing, future research, and collaboration on other topics that ultimately affect a culture of data sharing, as there remains a pressing need to generate high-quality evidence to support all that is done in clinical medicine. Although major work has been completed to actualize the vision of IPD sharing, there are still important action steps, identified in this paper, that must be addressed.
Ultimately, driven by a desire for high-quality science that enables new discoveries and dedicated individuals and institutions—and through continued engagement with the National Academy of Medicine, among other entities—we want to encourage individual investigators, regulators, scientists, and industry to continue to work to improve IPD sharing capabilities, with the belief that partnership and collaboration offers opportunity to advance science. We would also like to encourage federal and nonfederal funders to consider approaches for making funds available to support key data-sharing tasks, as outlined in Box 8. We hope that the action steps presented here will add to this effort and will reinforce the importance of robust clinical trial design and conduct.
Join the conversation!
![]() Tweet this! The ability to analyze individual patient-level data could contribute to better-informed health care decision-making and confidence in clinical trials. Read about a proposed strategy in our newest #NAMPerspectives: https://doi.org/10.31478/201906b
Tweet this! The ability to analyze individual patient-level data could contribute to better-informed health care decision-making and confidence in clinical trials. Read about a proposed strategy in our newest #NAMPerspectives: https://doi.org/10.31478/201906b
![]() Tweet this! Being able to utilize individual patient-level data could advance health and medicine – but significant barriers remain, including data security and anonymity. A new #NAMPerspectives reflects on how these barriers can be overcome: https://doi.org/10.31478/201906b
Tweet this! Being able to utilize individual patient-level data could advance health and medicine – but significant barriers remain, including data security and anonymity. A new #NAMPerspectives reflects on how these barriers can be overcome: https://doi.org/10.31478/201906b
![]() Tweet this! Our newest #NAMPerspectives discussion paper analyzes six case examples of individual patient-level data sharing exchanges, identifies their successes, and considers how to overcome their weaknesses: https://doi.org/10.31478/201906b
Tweet this! Our newest #NAMPerspectives discussion paper analyzes six case examples of individual patient-level data sharing exchanges, identifies their successes, and considers how to overcome their weaknesses: https://doi.org/10.31478/201906b
Download the graphics below and share them on social media!


References
- Institute of Medicine. 2010. Clinical data as the basic staple of health learning: Creating and protecting a public good: workshop summary. Washington, DC: The National Academies Press. https://doi.org/10.17226/12212
- Zarin, D. A., and T. Tse. 2016. Sharing individual participant data (IPD) within the context of the trial reporting system (TRS). PLOS Medicine 13(1):e1001946. https://doi.org/10.1371/journal.pmed.1001946
- Rathore, S. S., J. P. Curtis, Y. Wang, M. R. Bristow, and H. M. Krumholz. 2003. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 289(7):871-878.
- Rathore, S. S., Y. Wang, and H. M. Krumholz. 2002. Sex-based differences in the effect of digoxin for the treatment of heart failure. The New England Journal of Medicine 347(18):1403-1411. https://doi.org/10.1056/NEJMoa021266
- Ross, J. S., D. Madigan, M. A. Konstam, D. S. Egilman, and H. M. Krumholz. 2010. Persistence of cardiovascular risk after rofecoxib discontinuation. Archives of Internal Medicine 170(22):2035-2036. https://doi.org/10.1001/archinternmed.2010.461
- Naudet, F., C. Sakarovitch, P. Janiaud, I. Cristea, D. Fanelli, D. Moher, and J. P. A. Ioannidis. 2018. Data sharing and reanalysis of randomized controlled trials in leading biomedical journals with a full data sharing policy: Survey of studies published in The BMJ and PLOS Medicine. BMJ 360:k400. https://doi.org/10.1136/bmj.k400
- McKiernan, E. C., P. E. Bourne, C. T. Brown, S. Buck, A. Kenall, J. Lin, D. McDougall, B. A. Nosek, L. Ram, C. K. Soderberg, J. R. Spies, K. Thaney, A. Updegrove, K. H. Woo, and T. Yarkoni. 2016. How open science helps researchers succeed. Elife 5:e16800.
- Institute of Medicine. 2015. Sharing clinical trial data: Maximizing benefits, minimizing risk. Washington, DC: The National Academies Press. https://doi.org/10.17226/18998
- Ohmann, C., R. Banzi, S. Canham, S. Battaglia, M. Matei, C. Ariyo, L. Becnel, B. Bierer, S. Bowers, L. Clivio, M. Dias, C. Druml, H. Faure, M. Fenner, J. Galvez, D. Ghersi, C. Gluud, T. Groves, P. Houston, G. Karam, D. Kalra, R. L. Knowles, K. Krleža-Jerić, C. Kubiak, W. Kuchinke, R. Kush, A. Lukkarinen, P. S. Marques, A. Newbigging, J. O’Callaghan, P. Ravaud, I. Schlünder, D. Shanahan, H. Sitter, D. Spalding, C. Tudur-Smith, P. van Reusel, E. B. van Veen, G.
R. Visser, J. Wilson, and J. Demotes-Mainard. 2017. Sharing and reuse of individual participant data from clinical trials: Principles and recommendations. BMJ Open 7(12):e018647. https://doi.org/10.1136/bmjopen-2017-018647 - PLoS Medicine editors. 2016. Can data sharing become the path of least resistance? PLOS Medicine 13(1):e1001949. https://doi.org/10.1371/journal.pmed.1001949
- Tudur Smith, C., C. Hopkins, M. R. Sydes, K. Woolfall, M. Clarke, G. Murray, and P. Williamson. 2015. How should individual participant data (IPD) from publicly funded clinical trials be shared? BMC Medicine 13(1):298. https://doi.org/10.1186/s12916-015-0532-z
- Ohmann, C., S. Canham, R. Banzi, W. Kuchinke, and S. Battaglia. 2018. Classification of processes involved in sharing individual participant data from clinical trials. F1000Research 7:138. https://doi.org/10.12688/f1000research.13789.2
- Rowhani-Farid, A., and A. G. Barnett. 2016. Has open data arrived at the British Medical Journal (BMJ)? An observational study. BMJ Open 6(10). https://doi.org/10.1136/bmjopen-2016-011784
- Gamble, C., A. Krishan, D. Stocken, S. Lewis, E. Juszczak, C. Dore, P. R. Williamson, D. G. Altman, A. Montgomery, P. Lim, J. Berlin, S. Senn, S. Day, Y. Barbachano, and E. Loder. 2017. Guidelines for the content of statistical analysis plans in clinical trials. JAMA 318(23):2337-2343. https://doi.org/10.1001/jama.2017.18556
- Gibson, C. 2018. Moving from hope to hard work in data sharing. JAMA Cardiology 3(9):795-796. https://doi.org/10.1001/jamacardio.2018.0130
- Gay, H. C., A. S. Baldridge, and M. D. Huffman. 2018. Different population and end point definitions in reproduction analysis based on shared data—reply. JAMA Cardiology 3(9):894. https://doi.org/10.1001/jamacardio.2018.1791
- Natale, A., R. Stagg, and B. Zhang. 2018. Different population and end point definitions in reproduction analysis based on shared data. JAMA Cardiology 3(9):893-894. https://doi.org/10.1001/jamacardio.2018.1788
- Wilkinson, M. D., M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J. W. Boiten, L. B. da Silva Santos, P. E. Bourne, J. Bouwman, A. J. Brookes, T. Clark, M. Crosas, I. Dillo, O. Dumon, S. Edmunds, C. T. Evelo, R. Finkers, A. Gonzalez-Beltran, A. J. Gray, P. Groth, C. Goble, J. S. Grethe, J. Heringa, P. A. ‘t Hoen, R. Hooft, T. Kuhn, R. Kok, J. Kok, S. J. Lusher, M. E. Martone, A. Mons, A. L. Packer, B. Persson, P. Rocca-Serra, M. Roos, R.
van Schaik, S. A. Sansone, E. Schultes, T. Sengstag, T. Slater, G. Strawn, M. A. Swertz, M. Thompson, J. van der Lei, E. van Mulligen, J. Velterop, A. Waagmeester, P. Wittenburg, K. Wolstencroft, J. Zhao, and B. Mons. 2016. The FAIR guiding principles for scientific data management and stewardship. Scientific Data 3:160018. https://doi.org/10.1038/sdata.2016.18 - International Committee of Medical Journal Editors. 2018. Clinical trials: Registration and data sharing. Available at: http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html#two (accessed June 13, 2019).
- Bonini, S., H. G. Eichler, N. Wathion, and G. Rasi. 2014. Transparency and the European Medicines Agency—sharing of clinical trial data. The New England Journal of Medicine 371(26):2452-2455. https://doi.org/10.1056/NEJMp1409464
- External guidance on the implementation of the European Medicines Agency policy on the publication of clinical data for medicinal products for human use. 2017. The Netherlands: European Medicines Agency.
- Food and Drug Administration Amendments Act of 2007, Pub. L. No. 110-85.
- Moorthy, V. S., G. Karam, K. S. Vannice, and M. P. Kieny. 2015. Rationale for WHO’s new position calling for prompt reporting and public disclosure of interventional clinical trial results. PLOS Medicine 12(4):e1001819. https://doi.org/10.1371/journal.pmed.1001819
- National Institutes of Health. 2016. NIH policy on the dissemination of NIH-funded clinical trial information. Available at: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-149.html (accessed June 13, 2019).
- US Department of Health and Human Services. 2016. 42 CFR Part 11: Clinical trials registration and results information submission; final rule. Federal Register 81(183):64982-65157.
- US Department of Veterans Affairs. 2018. ORD sponsored clinical trials: Registration and submission of summary results. Available at: https://www.research.va.gov/resources/ORD_Admin/clinical_trials (accessed June 13, 2019).
- World Medical Association. 2013. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20):2191-2194. https://doi.org/10.1001/jama.2013.281053
- US Food and Drug Administration. 2018. Study data for submission to CDER and CBER. Available at: https://www.fda.gov/ForIndustry/DataStandards/StudyDataStandards/ucm587508.htm (accessed June 13, 2019).
- McCall, B. 2018. What does the GDPR mean for the medical community? Lancet 391(10127):1249-1250. https://doi.org/10.1016/S0140-6736(18)30739-6
- Krumholz, H. M., C. P. Gross, K. L. Blount, J. D. Ritchie, B. Hodshon, R. Lehman, and J. S. Ross. 2014. Sea change in open science and data sharing: Leadership by industry. Circulation: Cardiovascular Quality and Outcomes 7(4):499-504. https://doi.org/10.1161/circoutcomes.114.001166
- AACR Project GENIE Consortium. 2017. AACR project GENIE: Powering precision medicine through an international consortium. Cancer Discovery 7(8):818-831. https://doi.org/10.1158/2159-8290.CD-17-0151
- Coady, S. A., G. A. Mensah, E. L. Wagner, M. E. Goldfarb, D. M. Hitchcock, and C. A. Giff en. 2017. Use of the National Heart, Lung, and Blood Institute data repository. The New England Journal of Medicine 376(19):1849-1858. https://doi.org/10.1056/NEJMsa1603542
- Duke Clinical Research Institute. 2018. SOAR data: Available datasets: Duke cardiac catheterization datasets. Available at: https://dcri.org/our-approach/data-sharing/soar-data (accessed June 13, 2019).
- Krumholz, H. M., and J. Waldstreicher. 2016. The Yale Open Data Access (YODA) Project—a mechanism for data sharing. The New England Journal of Medicine 375(5):403-405. https://doi.org/10.1056/NEJMp1607342
- National Institutes of Health. 2017. NIH data sharing repositories. Available at: https://www.nlm.nih.gov/NIHbmic/nih_data_sharing_repositories.html (accessed June 13, 2019).
- Springer Nature Limited. 2018. Recommended data repositories. Available at: https://nature.com/sdata/policies/repositories (accessed June 13, 2019).
- Strom, B. L., M. Buyse, J. Hughes, and B. M. Knoppers. 2014. Data sharing, year 1—access to data from industry-sponsored clinical trials. The New England Journal of Medicine 371(22):2052-2054. https://doi.org/10.1056/NEJMp1411794
- Broes, S., D. Lacombe, M. Verlinden, and I. Huys. 2018. Toward a tiered model to share clinical trial data and samples in precision oncology. Frontiers in Medicine 5:6. https://doi.org/10.3389/fmed.2018.00006
- Sydes, M. R., A. L. Johnson, S. K. Meredith, M. Rauchenberger, A. South, and M. K. Parmar. 2015. Sharing data from clinical trials: The rationale for a controlled access approach. Trials 16(1):104. https://doi.org/10.1186/s13063-015-0604-6
- Coady, S. A., and E. Wagner. 2013. Sharing individual level data from observational studies and clinical trials: A perspective from NHLBI. Trials 14:201. https://doi.org/10.1186/1745-6215-14-201
- Giff en, C. A., L. E. Carroll, J. T. Adams, S. P. Brennan, S. A. Coady, and E. L. Wagner. 2015. Providing contemporary access to historical biospecimen collections: Development of the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Biopreservation Biobank 13(4):271-279. https://doi.org/10.1089/bio.2014.0050
- Ross, J. S., J. D. Ritchie, E. Finn, N. R. Desai, R. L. Lehman, H. M. Krumholz, and C. P. Gross. 2016. Data sharing through an NIH central database repository: A cross-sectional survey of BioLINCC users. BMJ Open 6(9):e012769. https://doi.org/10.1136/bmjopen-2016-012769
- Navar, A., M. J. Pencina, J. A. Rymer, D. M. Louzao, and E. D. Peterson. 2016. Use of open access platforms for clinical trial data. JAMA 315(12):1283-1284. https://doi.org/10.1001/jama.2016.2374
- The YODA Project. Submitted requests to use Johnson & Johnson data. Aavilable at: https://yoda.yale.edu/metrics/submitted-requests-use-johnson-johnson-data (accessed June 13, 2019).
- Zhu, C. S., P. F. Pinsky, J. E. Moler, A. Kukwa, J. Mabie, J. M. Rathmell, T. Riley, P. C. Prorok, and C. D. Berg. 2017. Data sharing in clinical trials: An experience with two large cancer screening trials. PLOS Medicine 14(5):e1002304. https://doi.org/10.1371/journal.pmed.1002304
- US Food and Drug Administration. 2019. Study data standards resources. Available at: https://www.fda.gov/ForIndustry/DataStandards/StudyDataStandards/default.htm#Catalog (accessed June 13, 2019).
- US Food and Drug Administration. 2014. Providing regulatory submissions in electronic format—standardized study data. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm292334.pdf (accessed June 13, 2019).
- Budin-Ljosne, I., J. Isaeva, B. M. Knoppers, A. M. Tasse, H. Y. Shen, M. I. McCarthy, and J. R. Harris. 2014. Data sharing in large research consortia: Experiences and recommendations from ENGAGE. European Journal of Human Genetics 22(3):317-321. https://doi.org/10.1038/ejhg.2013.131
- Hickner, J., and L. A. Green. 2015. Practice-based research networks (PBRNs) in the United States: Growing and still going after all these years. The Journal of the American Board of Family Medicine 28(5):541-545. https://doi.org/10.3122/jabfm.2015.05.150227
- Poldrack, R. A., and K. J. Gorgolewski. 2014. Making big data open: Data sharing in neuroimaging. Nature Neuroscience 17(11):1510-1517. https://doi.org/10.1038/nn.3818
- van Panhuis, W. G., P. Paul, C. Emerson, J. Grefenstette, R. Wilder, A. J. Herbst, D. Heymann, and D. S. Burke. 2014. A systematic review of barriers to data sharing in public health. BMC Public Health 14:1144. https://doi.org/10.1186/1471-2458-14-1144
- Bierer, B. E., M. Crosas, and H. H. Pierce. 2017. Data authorship as an incentive to data sharing. The New England Journal of Medicine 376(17):1684-1687. https://doi.org/10.1056/NEJMsb1616595
- Peterson, E. D., and F. W. Rockhold. 2018. Finding means to fulfill the societal and academic imperative for open data access and sharing. JAMA Cardiology 3(9):793-794. https://doi.org/10.1001/jamacardio.2018.0129
- Silva, L. 2014. PLOS’ new data policy: Public access to data. EveryONE: PLOS ONE Community Blog, February 24. Available at: http://blogs.plos.org/everyone/2014/02/24/plos-new-data-policy-public-access-data-2 (accessed June 13, 2019).
- Wilhelm, E. E., E. Oster, and I. Shoulson. 2014. Approaches and costs for sharing clinical research data. JAMA 311(12):1201-1202. https://doi.org/10.1001/jama.2014.850
- Canham, S., and C. Ohmann. 2016. A metadata schema for data objects in clinical research. Trials 17(1):557. https://doi.org/10.1186/s13063-016-1686-5
- Lo, B., and D. L. DeMets. 2016. Incentives for clinical trialists to share data. The New England Journal of Medicine 375(12):1112-1115. https://doi.org/10.1056/NEJMp1608351
- Berger, M. L., H. Sox, R. J. Willke, D. L. Brixner, H. G. Eichler, W. Goettsch, D. Madigan, A. Makady, S. Schneeweiss, R. Tarricone, S. V. Wang, J. Watkins, and C. D. Mullins. 2017. Good practices for realworld data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiology Drug Safety 26(9):1033-1039. https://doi.org/10.1002/pds.4297
- Wang, S. V., S. Schneeweiss, M. L. Berger, J. Brown, F. de Vries, I. Douglas, J. J. Gagne, R. Gini, O. Klungel, C. D. Mullins, M. D. Nguyen, J. A. Rassen, L. Smeeth, and M. Sturkenboom. 2017. Reporting to improve reproducibility and facilitate validity assessment for healthcare database studies v1.0. Pharmacoepidemiology Drug Safety 26(9):1018-1032. https://doi.org/10.1002/pds.4295