Expanding the Role of N-of-1 Trials in the Precision Medicine Era: Action Priorities and Practical Considerations

Heterogeneous responses to the same therapy among individual patients are commonplace in both biomedical research and patient care. Despite being considered by many as the pinnacle of the evidence-based medicine hierarchy, parallel group randomized clinical trials provide limited help with this routine quandary. Thus, clinicians following evidence-based treatment guidelines are often left to their own devices to tackle heterogeneity in both treatment efficacy and side effects. Clinicians have to make their best guesses on the likely response of a patient based on the average response from the participants in clinical trials. This extrapolation is hardly personalized, precise, or data-driven [1].
At its core, precision medicine seeks solutions to such challenges. Personalized trial designs, also known as N-of-1 trials, have been developed to address this fundamental problem but are not yet part of the arsenal of precision medicine. Currently, these designs are rarely used in clinical practice, in evidence-genesis, or in the formation of guidelines. In this commentary, we articulate the rationale for wider use of these methods for managing chronically ill and behaviorally challenged patients; offer observations about why this approach foundered but is opportune now; and suggest a road map of action priorities to accomplish precision medicine’s vision for identifying the best treatments for each patient.
What are N-of-1 Trials?
N-of-1 trials belong to a family of single-subject clinical trial designs that aim to determine how a patient responds to various treatment regimens (including dosage). The most common form of N-of-1 trials uses a multiple crossover design; multiple exposures to reversible treatments are given in a random order, and the patient’s response to each treatment can be compared with each of his or her other responses. In other words, time periods of treatment exposure are randomized, rather than patients [2]. Similar to parallel group trials, these trials can be masked or blinded, have a random allocation of interventions, have multiple active comparators, and include a placebo or usual care comparator. The conduct of N-of-1 trials also includes rigorous assessment of treatment outcomes and adverse effects, a priori hypotheses, and statistical analyses. This enables patients and their clinicians to determine the relative benefits and harms of possible treatments that matter to them [3]. This approach differs from the typical approach adopted by clinicians to determine the optimal treatment for their patients. In contrast to the regimented and objective method of N-of-1 trials, “trials of therapies” are usually informal, employ a single treatment at a time, assess the response informally, and determine which treatment is “successful”.
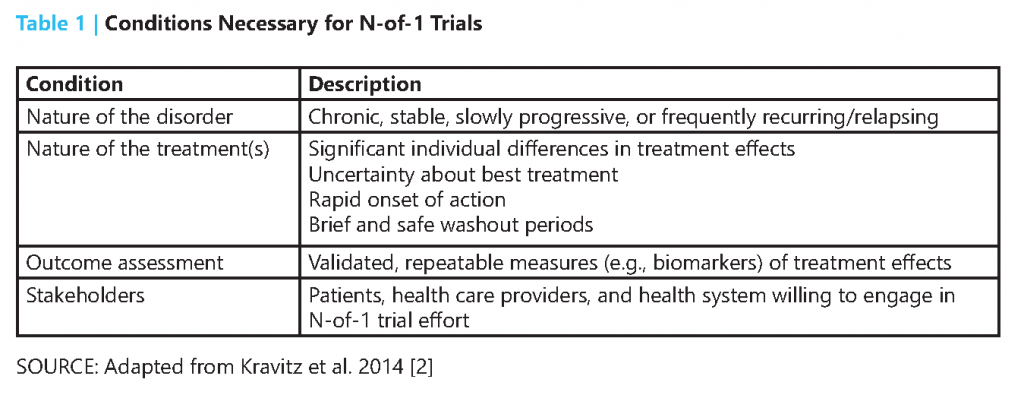
N-of-1 trials are indicated only when certain conditions are met (Table 1). Because of the multiple crossover design, N-of-1 trials typically cannot be used for acute conditions or rapidly progressive diseases. Instead, N-of-1 trials are most applicable to chronic conditions that have measurable markers for treatment effectiveness or adverse effects, e.g., symptoms or biomarkers [2]. Treatment assessed in N-of-1 trials should ideally have rapid onset of action, as well as a short washout period to ensure few carryover effects when a new treatment is tested [3].
These trials bring the most value to patient-centered care when there is substantial uncertainty surrounding the comparative effectiveness and adverse effects across multiple treatment options for an individual patient [2]. Although one source of uncertainty is the heterogeneity of treatment effects, uncertainty can also result from the lack of relevant, parallel-group clinical trials, conflicting evidence, or limited generalizability to the patient at hand. In addition, N-of-1 trials can be useful when a new therapy demonstrates marginal benefits over existing treatments but with likely trade-offs based on cost or various side effects.
Personalized trials have led to informed changes in treatment, cessation of treatment, or confirmation of the efficacy of the original treatment [4]. For example, in one study of 71 N-of-1 trials for patients with chronic pain, 46 patients (65 percent) decided to change their pain medication based on the results [5]. Over the past 30 years, more than 2,000 patients have participated in published N-of-1 trials, and fewer than 10 percent of the participants chose treatments inconsistent with the results. [4]
Despite early promise, efforts to implement N-of-1 trials have faltered. To date, personalized trials have largely been conducted in academic settings or through grant-funded clinical research. Other than isolated pockets of activity, these trials are conducted infrequently and are far from standard practice in clinical medicine. For example, the method has not been widely included in the evidence-based medicine curriculum and remains unknown to many. Another reason N-of-1 trials have not been widely adopted may be that they are insufficiently appealing to patients or clinicians to justify the cost and effort needed to design and implement them. Further, the methodology and use cases of N-of-1 trials are often misunderstood or implemented incorrectly. These factors undermine the momentum for incorporating such an approach more widely in standard clinical care to improve quality, outcomes, and patient-centered care delivery.
Renewed Momentum in the Precision Medicine Era
Over the last decade, the movement toward precision medicine and patient-centered health care has renewed enthusiasm for N-of-1 trials. Advances in electronic health records and mobile health technology further enhance the technological underpinning for embedding more rigorous approaches to assess treatment outcomes and to optimize treatment plans for each patient [6]. Experts increasingly suggest that precision health care may be achieved at scale by leveraging information technology, creating defined patient profiles, and applying “mass customization” strategies. Such strategies are widely used in business sectors to better connect products to specific customer needs. Offering options for implementing personalized trials at points of care with minimum additional effort would allow patients and clinicians to more quickly design and implement their own trials. From a health care system standpoint, providing more customized, precise patient-centered care offers the potential to simultaneously improve care quality, reduce inefficiencies, and promote continuous learning toward high-value care.
Action Priorities for Expanding the Role of Personalized Trials
To broaden the role of personalized trials and more properly embed them within our evidence-genesis tools, we propose four action priorities for the field (see Table 2).
First, systematically define high-impact, high-demand clinical areas for personalized trials. Although several areas have been suggested based on past experience, there has not been a systematic scan to identify a comprehensive list of clinical use cases. The clinical areas that could benefit most from N-of-1 trials likely involve the management of high-burden, high-prevalence, high-cost disorders or symptoms, such as chronic pain, diabetes, arthritis, depression, obesity, smoking, dementia, mild hypothyroidism, hypertension, generic versus trade name medication use, asthma, hyperlipidemia, and insomnia.
Second, build personalized trial implementation platforms and resources. Currently, there are few resources and software tools designated for conducting, managing, and analyzing personalized trials. For clinicians interested in embedding N-of-1 trials in their clinical practice, a personalized trial platform needs to be developed that allows users to customize trial designs according to the use case. A shared service that delivers custom-built trial prototypes, uses a dedicated pharmacy, and facilitates data collection and analyses might best reduce logistical and cost barriers to widespread implementation. Over time, such infrastructure can foster the development of successful supporting services and mobile health applications that both facilitate N-of-1 trials and reduce technical barriers and implementation costs.
Third, form multi-stakeholder collaboratives to inform best practices and policies. Although personalized trial methods are somewhat mature from a research standpoint, integration into clinical practice requires substantial further work. A better understanding of the circumstances under which patients would be interested in personalized trials will also foster better understanding of the high-impact areas [2]. Issues such as best practices in consent, privacy protections, and data portability remain unsolved. Further, health care delivery organizations will also be interested in questions of cost, liability, quality reporting, and reimbursement mechanisms. The policy and business landscapes remain wide open. Even for those who wish to accelerate the adoption of personalized trials to improve care, a strategic alliance that allows diverse stakeholder input—especially those of patients—is necessary to lay the groundwork.
Fourth, we need to construct an open, transparent, deep phenotype data bank, where N-of-1 trial data can be deposited. Pooling N-of-1 trials quantitatively could provide intriguing comparative effectiveness insights in an approach that is more efficient than conventional between-group randomized controlled trials. Scientists could derive phenotypes empirically from N-of-1 trial data and better understand the uniqueness of therapy responders versus nonresponders. Off-target therapeutic responses could also be mined in this database, because the unique responsiveness of one patient to several time periods of therapy exposure would be available.
Conclusion
With a focus on determining the right therapy that optimizes the outcomes and values meaningful to each individual patient, personalized trials can help patients and clinicians make decisions that are informed by high-integrity, evidence-based information uniquely relevant to the single, specific patient—the one in front of the clinician right now. In this era of tremendous advances in technology, it is critical to energize our investment in providing the right care, at the right time, to the right patient. Despite a growing interest in the use of N-of-1 trials to guide care management, more work is needed to engage stakeholders across the health care ecosystem to inform the use cases and the value proposition for personalized trials. The inclusion of patients, primary care providers, insurers, governmental agencies, and industry has to co-occur when designing, evaluating, and incorporating personalized trials into clinical care service, into our evidence base, and into the precision medicine movement.
Join the conversation!
![]() Tweet this! New from @theNAMedicine: Expanding the Role of N-of-1 Trials in the Precision Medicine Era: Priorities and Practical Considerations: https://doi.org/10.31478/201812d #NAMPerspectives
Tweet this! New from @theNAMedicine: Expanding the Role of N-of-1 Trials in the Precision Medicine Era: Priorities and Practical Considerations: https://doi.org/10.31478/201812d #NAMPerspectives
![]() Tweet this! N-of-1 trials are far from standard practice in medicine – but wider implementation of these individualized trials could move care toward precision health: https://doi.org/10.31478/201812d #NAMPerspectives
Tweet this! N-of-1 trials are far from standard practice in medicine – but wider implementation of these individualized trials could move care toward precision health: https://doi.org/10.31478/201812d #NAMPerspectives
![]() Tweet this! High-burden, high-prevalence, and high-cost disorders could benefit most from the results of N-of-1 trials, leading to more effective and efficient care: https://doi.org/10.31478/201812d #NAMPerspectives
Tweet this! High-burden, high-prevalence, and high-cost disorders could benefit most from the results of N-of-1 trials, leading to more effective and efficient care: https://doi.org/10.31478/201812d #NAMPerspectives
![]() Tweet this! N-of-1 trials may provide data that allow clinicians to craft individual treatment plans for the most important patient: the one in front of them: https://doi.org/10.31478/201812d #NAMPerspectives
Tweet this! N-of-1 trials may provide data that allow clinicians to craft individual treatment plans for the most important patient: the one in front of them: https://doi.org/10.31478/201812d #NAMPerspectives
![]() Tweet this! Implementation of N-of-1 trials to provide insight into the needs of specific patients could contribute to precision medicine, but requires the engagement of interdisciplinary stakeholders: https://doi.org/10.31478/201812d #NAMPerspectives
Tweet this! Implementation of N-of-1 trials to provide insight into the needs of specific patients could contribute to precision medicine, but requires the engagement of interdisciplinary stakeholders: https://doi.org/10.31478/201812d #NAMPerspectives
Download the graphics below and share them on social media!


References
- Schork, N. J. 2015. Personalized medicine: Time for one-person trials. Nature 520:609-611. Available at: https://www.nature.com/news/personalized-medicine-time-for-one-person-trials-1.17411 (accessed September 2, 2020).
- Kravitz R., and N. Duan, eds., and the DEcIDE Methods Center N-of-1 Guidance Panel (N. Duan, I. Eslick, N. B. Gabler, H. C. Kaplan, R. L. Kravitz, E. B. Larson, W. D. Pace, C. H. Schmid, I. Sim, and S. Vohra). 2014. Design and implementation of N-of-1 trials: A user’s guide. AHRQ Publication No. 13 (14)-EHC122-EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: https://eff ectivehealthcare.ahrq.gov/topics/n-1-trials/research-2014-5 (accessed September 2, 2020).
- Guyatt, G., D. Sackett, J. Adachi, R. Roberts, J. Chong, D. Rosenbloom, and J. Keller. 1988. A clinician’s guide for conducting randomized trials in individual patients. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne 139:497-503. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1268200/ (accessed September 2, 2020)
- Mirza, R., S. Punja, S. Vohra, and G. Guyatt. 2017. The history and development of N-of-1 trials. Journal of the Royal Society of Medicine 110:330-340. https://doi.org/10.1177/0141076817721131
- Nikles, C. J., M. Yelland, P. P. Glasziou, and C. Del Mar. 2005. Do individualized medication effectiveness tests (n-of-1 trials) change clinical decisions about which drugs to use for osteoarthritis and chronic pain? American Journal of Therapeutics 12:92-97. https://doi.org/0.1097/00045391-200501000-00012
- Council, N. R. 2011. Toward precision medicine: Building a knowledge network for biomedical research and a new taxonomy of disease. Washington, DC: National Academies Press. Available at: https://pubmed.ncbi.nlm.nih.gov/22536618/ (accessed September 2, 2020).