Dismantling Buprenorphine Policy Can Provide More Comprehensive Addiction Treatment

Those working on changing the course of the opioid crisis cannot afford to overlook simple solutions that could bring timely, positive results. Changing the policies that impact buprenorphine prescribing could have immediate impacts on access to opioid addiction treatment, resulting in fewer opioid-related deaths and decreasing the economic burden of this crisis by billions of dollars. Over 49,000 people died from opioid-related overdose in 2017 and the financial cost of the opioid crisis is estimated to be 500 billion dollars per year. [1,2] The vast majority of these costs are from opioid-related deaths. [1] Buprenorphine, often prescribed in the brand name Suboxone, is one of three FDA-approved medication treatment options for opioid use disorder (OUD), but buprenorphine is currently not available to all who could benefit from it. This commentary will evaluate the Drug Addiction Treatment Act (DATA) of 2000 [3] and unpack how timely action toward dismantling legislative restrictions on buprenorphine prescribing could be a powerful step towards ensuring increased access to treatment of OUD in America.
Why is Buprenorphine Access So Critical?
Buprenorphine is an FDA-approved first-line treatment for OUD that saves lives. [4] Buprenorphine is a partial agonist of opioid receptors in the brain, which differentiates it from methadone, oxycodone, heroin, and fentanyl, which are full agonists. [4] Unlike these other opioids, buprenorphine has a ceiling effect— in other words, after a certain dose, there is no additional effect, thereby significantly reducing the risk of overdose as compared to other opioids. Buprenorphine binds very tightly to the opioid receptors, blocking other opioids that do not have as much affinity, such as heroin. One of the most common formulations of buprenorphine is combined with naloxone, which prevents overdose if the combination product is injected, thereby reducing misuse. The naloxone in the formulation was added as an “abuse deterrent” and does not have any effect if the medication is taken appropriately. [6]
Buprenorphine is an effective and safe medication to treat OUD and is classified as a Schedule III drug. Maintenance treatment with opioid agonists, like buprenorphine and methadone, drastically reduces the risk of fatal overdose and reduces both withdrawal symptoms and opioid cravings. [5] Buprenorphine treatment is also associated with long term benefits such as increased retention in treatment, decreased illicit opioid use, and decreased behaviors associated with the transmission of HIV and Hepatitis C. [4] However, misinformation and distrust of the evidence supporting the efficacy of these medications still circulate. [4,5] False beliefs that taking prescribed buprenorphine is “substituting one drug for another” and that patients are likely to sell their prescription has made many providers hesitant to prescribe this life-saving medication and policy-makers reluctant to allow expanded access to treatment. [4,7]
The Drug Addiction Treatment Act of 2000
In 2000, Congress established DATA to expand access to treatment for OUD by allowing doctors to prescribe approved Schedule III through V medications to treat OUD in an outpatient setting. DATA was conceived and passed to allow doctors to more freely treat their patients with OUD with approved medications and expand access to medical treatment for patients with addiction. [7] Prior to this legislation, the only options for medication for OUD treatment (and still the only option for methadone treatment) included doctors seeking prior approval from the Drug Enforcement Administration (DEA) or having to be affiliated with a freestanding opioid treatment program (OTP). After DATA’s passing, trained and approved doctors could be formally certified to treat patients by prescribing Schedule III-V medications that have been approved for OUD treatment in an outpatient setting. To date, the only medication being prescribed through this certification mechanism is buprenorphine. [3] The permit from this certification is commonly referred to as the “X-waiver.”
The passage of DATA did have some initial positive outcomes. One of its goals was to increase the availability of medication for addiction treatment by allowing primary care doctors to prescribe these medications in their established practices. Allowing buprenorphine treatment in an outpatient office (as was possible after passage of DATA) was groundbreaking in terms of access because patients could be treated for OUD by their primary care provider, addiction physician specialist, or psychiatrist in settings other than an OTP. A 2006 Substance Abuse and Mental Health Services Administration report demonstrated that access to these medications increased after the passage of DATA, including in geographic areas that previously did not have adequate addiction services. [8] Today, office-based treatment is a successful and feasible model for OUD treatment, but has not been able to keep up with demand.
The DATA legislation was prompted by buprenorphine’s impending approval by the U.S. Food and Drug Administration for OUD treatment. In hearings on this bill, the Congressional Budget Office estimated that only 100,000 individuals would benefit from buprenorphine treatment for OUD. [9] The legislation limited physicians to prescribing buprenorphine to 30 patients at any time–the only medication with such a limit. The DATA legislation was released with patient limits due to concerns about adverse public health events, including potential diversion (selling or buying) and misuse of buprenorphine. These patient limits remain today, although two amendments to the rule raised the cap to 100 after one year of prescribing, then to 275 for a select group of physicians specialized in addiction medicine. Paradoxically, buprenorphine, when prescribed for pain, is not subjected to these limits and
can be prescribed by physicians and advanced practitioners without an X-waiver.
Most notably, current limits on how many patients a clinician can treat still underestimate the overall treatment needs in 2019. The nature of OUD is vastly different today compared to when the X-waiver system was created in 2000. As of 2019, only approximately 35 percent of people who have OUD receive addiction treatment, leaving an estimated 2.2 million people untreated. Additionally, the vast majority of licensed addiction treatment programs do not offer FDA-approved medications. [10] When DATA was passed, legislators were not facing the same crisis that exists today, and the policy has not kept pace with the exponential growth and lethality of the overdose crisis.
Why is There a Treatment Gap?
Addiction treatment has historically been separated from the medical and psychiatric fields, relying on specialized doctors and programs operating outside of the traditional medical system. DATA sought to change that paradigm. However, the demand for addiction treatment has greatly outpaced the number of waivered physicians and has not been adequately met by treatment at OTPs. [11] Policies that address access to and expansion of OTPs should also be explored, but are outside the scope of this commentary.
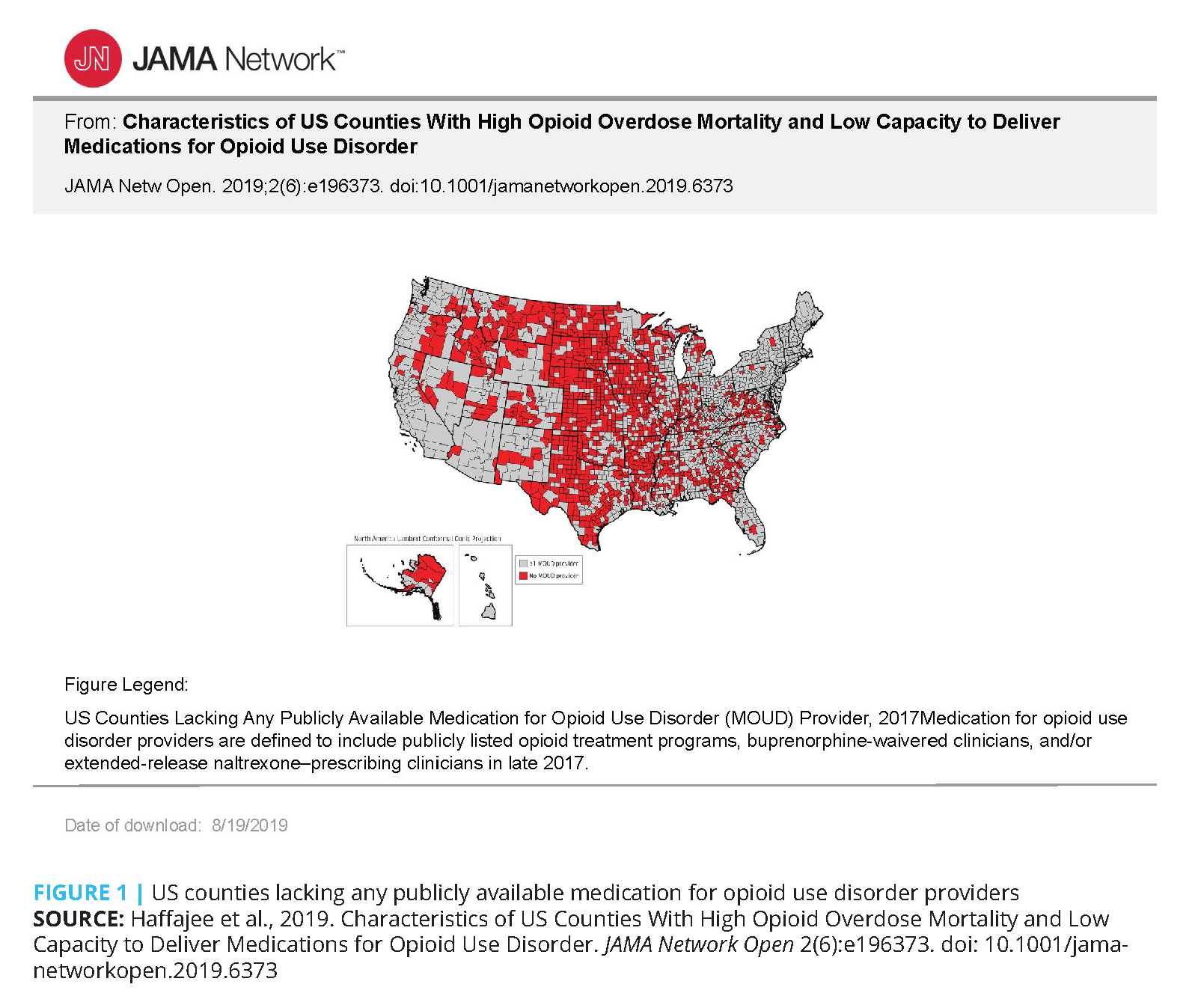
Most physicians do not have an X-waiver, and many who do are treating fewer patients than they can per the waiver limit. [12] Many areas of the country do not have any X-waivered prescribers at all, which means that this treatment is not available for people with OUD in those geographic regions. Approximately half of all rural counties still lack a provider waivered to prescribe buprenorphine, leaving millions of residents without access to this, and usually any other, medication treatment for OUD (see Figure 1). [12] Researchers have identified Appalachia, the Midwest, and Rocky Mountain areas as the most at risk for opioid overdose because they are devoid of adequate treatment access. [12]
Policy makers have attempted to expand access to buprenorphine with the X-waiver in place. The Comprehensive Addiction and Recovery Act of 2016 aimed to expand treatment capacity by allowing nurse practitioners and physician assistants to become eligible for the X-waiver. [13] In that same year, the U.S. Department of Health and Human Services raised the patient caps for addiction physician specialists and for others working in qualified practice settings. While these statutory and regulatory steps intended to expand access, they were not sufficient in meeting the demand and there is no definitive evidence that these expansions have made a significant impact on the treatment gap.
Provider Barriers to Prescribing Buprenorphine
There are a number of reasons providers are unwilling to prescribe buprenorphine. Surveys of doctors with and without waivers reported insufficient education on how to recognize and treat OUD, lack of institutional and clinician support, poor care coordination, provider stigma, financial concerns and regulatory hurdles as primary reasons why they did not seek a waiver or, if they did have a waiver, did not prescribe up to their patient limits. [14] The data strongly suggest that educating clinicians about treating addiction as a disease is imperative to integrating buprenorphine into their practices. Many doctors report that they are not educated enough to comfortably prescribe buprenorphine for addiction. [14]
Currently, to obtain an X-waiver, physicians must take an eight-hour training course on addiction treatment, controlled substance regulations, and buprenorphine prescribing. The regulatory barriers imposed by the X-waiver, specifically regular DEA audits and potential law enforcement surveillance, intimidate some clinicians. Random checks on X-waivered doctors can be anxiety-provoking and an additional disruption to their already packed schedules, which can lead to doctors not pursuing a waiver or to stop prescribing buprenorphine after getting one. [14] These combined factors create a strong chilling effect where providers seek to minimize risk rather than engage in treating people with OUD. [15]
The rules established after the passing of the Comprehensive Addiction and Recovery Act required advanced practitioners such as nurse practitioners and physician assistants to undergo 24 hours of training to gain the ability to prescribe buprenorphine for treatment of OUD. Additionally, many states have policies that require nurse practitioners and physician assistants to have a collaborating physician who also has an X-waiver before they can obtain one. Such policies further inhibit their ability to provide buprenorphine treatment and are particularly problematic in rural areas that have health care provider shortages. Having nurse practitioners and physician assistants who are able to prescribe buprenorphine could be key in helping to further expand treatment access to people who have OUD. Reversing the trends of the opioid overdose epidemic will require as many qualified practitioners as are willing to help.
Effects on Public Health
Adverse public health outcomes, such as diversion (selling or misusing) of medications used to treat OUD were a major concern to policy makers when DATA was being debated. [7] The 2006 Substance Abuse and Mental Health Services Administration analysis found little evidence of widespread diversion of buprenorphine, but more recent analyses have found that buprenorphine is now more available through the black market. [16]
Buprenorphine diversion is strongly correlated with a lack of access to appropriate treatment in the community. [16] The increased demand for buprenorphine outside of formal treatment settings is largely due to people managing their own withdrawal from opioids and attempting to moderate other opioid use. [16] For example, people who do not have access to a medical provider able to prescribe buprenorphine or who are trying to “detox” on their own may choose to buy buprenorphine from a friend or someone on the street. Some of these themes are captured in the opening quote of this article from a person in recovery. Theoretically, the health care industry could absorb most of the diverted buprenorphine market by offering more low-barrier treatment options and flexible provider networks.
How to Move Forward?
Removing Barriers to Buprenorphine Treatment
Allowing buprenorphine to be prescribed without a separate license to treat addiction is not a new concept and has been used by many other countries to promote sustainable substance use treatment. Many countries— including Canada, France, and Switzerland—do not have a comparable restriction on buprenorphine and have not observed significant adverse public health outcomes.
France is often cited as an example of a case in which allowing doctors to freely prescribe buprenorphine led to a dramatic reduction in overdose deaths. In the late 1990s, France deregulated buprenorphine and the rate of opioid overdose decreased by a staggering 80 percent. [17] While it is unlikely that the U.S. would observe the same dramatic reduction (a conclusion based, in part, on the fact that America has a more lethal drug supply and fewer comprehensive safety net programs), the France example demonstrates the potential of widely-available buprenorphine to save tens of thousands of lives.
Patients should be able to ask the provider they know and trust to provide them with the treatment they need. In most settings when a patient with OUD asks a medical provider for treatment, the patient is given a list of numbers to call with little additional support. Instead, patients should be able to initiate buprenorphine treatment immediately, especially given the documented positive health outcomes related to its use and the safety profile of the medication. [4]
Every health care provider should be empowered to give patients the care they need if that care is both safe and reasonable. There are many medications that doctors can prescribe without additional training that are much more dangerous than buprenorphine, and timely treatment for OUD is far less complicated than treating the downstream effects of long-term, untreated OUD. Additionally, clinicians need education and clinical support to become confident and committed to providing this care—restrictions due to legislation and regulations impede that level of comfort and commitment.
Changing the Culture of Treatment in America
Stigma against people who use drugs is pervasive and exists in medical treatment settings. In a recent survey of over 500 physicians with and without the X-waiver, doctors without waivers cited a belief that becoming waivered would result in having too many patients that need buprenorphine and concerns about diversion. [14] The survey also revealed that many physicians that did not pursue a waiver held negative attitudes toward buprenorphine treatment and patients who need it. [14] Such findings are concerning, as these perceptions are not based in evidence and can be stigmatizing. The historical view that treating people with substance use disorders is not part of standard medical care is perpetuated by the carving out of regulations and processes for medications to treat addiction. To adequately expand access to evidence-based treatment for OUD, it is paramount that addiction treatment be seen as part of medical care for all patients. [19] Removing the X-waiver requirement and allowing buprenorphine to be treated like other Schedule III drugs is one way to address this.
The presence of the X-waiver creates a sense that prescribing buprenorphine to treat OUD must be extremely complicated or risky, as clinicians are required to obtain additional training to prescribe buprenorphine while they are allowed, without authorization, to prescribe opioids, including buprenorphine, for pain. Any clinician able to prescribe Schedule III drugs can be taught to initiate treatment for OUD with buprenorphine when indicated and to refer complex patients to specialists for a more intensive evaluation and services as needed. This is a common workflow in the medical field, but is complicated by only permitting only X-waivered clinicians to prescribe this life-saving care.
While eliminating the X-waiver requirements would allow any clinician (who is able to prescribe Schedule III medications) to prescribe buprenorphine, it would not guarantee they will feel comfortable doing so. Currently, medical, advance practice nursing, and physician assistant schools are not required to train students on addiction medicine and continuing education requirements do not necessarily require addiction training, making prescribing buprenorphine foreign to many prescribers.
Comprehensive training in addiction medicine needs to be integrated into health professional education if the field is to properly address the opioid crisis. Integration of addiction medicine training into all levels of education is needed to prepare a workforce to deal with this public health crisis. Including X-waiver education in DEA licensing requirements would better ensure that all DEA-licensed practitioners understand how to recognize OUDs and treat them with buprenorphine. Additionally, integrating addiction education into health professional curricula would allow clinicians to have a more complete and functional knowledge of addiction treatment. Keeping the X-waiver in place does not meaningfully address these educational needs.
Conclusion
DATA must be updated to address the overdose crisis that weighs so heavily on America in 2019. The last 20 years have seen millions of people suffer from untreated OUD and hundreds of thousands of preventable deaths. This regulation has not kept pace with the grim reality; it achieved the goals of the day, which were to expand access to medications for OUD outside of OTP settings and support additional effective treatment options. Innovative care models, including office-based addiction treatment, were created under this legislation. In time, DATA limited the expansion of treatment in communities across the country and placed regulatory burdens on the medical providers who would have otherwise been able to treat people with OUD. Eliminating the X-waiver could allow for an expansion in access to life-saving treatment across the country.
The authors of this commentary take seriously the potential downsides of eliminating the X-waiver. Currently, the X-waiver does serve as one source of education about addiction, but it would be best for these education gaps to be addressed in a more comprehensive manner. The additional education requirements provided in the X-waiver training should be absorbed in health professional schools’ curricula, which are already beginning to include addiction medicine in their training. Additionally, continuing education requirements for controlled substance and DEA licensure renewal should be expanded to include buprenorphine guidelines and information on prescribing, in addition to any pain treatment requirements.
Every clinician willing to prescribe buprenorphine should be encouraged to do so. When a clinician determines they can help, they should be able to treat patients and be limited only by their time and resources. The elimination of the X-waiver is an investment in treating the opioid crisis. Evidence-based treatment for OUD increases individuals’ chances of gainful employment, reduces criminal activity, and reduces the risk of exposure to HIV and Hepatitis C. [18] Repealing the X-waiver will start a domino effect of positive change. It sends a strong message that treating OUD is a part of common medical practice. With a death toll of 49,000 Americans a year from opioid overdose, there is no time to lose.
Join the conversation!
![]() Tweet this! Changing the policy around buprenorphine prescribing could have immediate and sweeping impacts on how addiction is treated in the U.S. Learn more in our newest #NAMPerspectives: https://doi.org/10.31478/201909a
Tweet this! Changing the policy around buprenorphine prescribing could have immediate and sweeping impacts on how addiction is treated in the U.S. Learn more in our newest #NAMPerspectives: https://doi.org/10.31478/201909a
![]() Tweet this! Many individuals who could benefit from buprenorphine – an FDA-approved treatment – are unable to receive it. Authors of this #NAMPerspectives commentary discuss why regulations must keep pace with need: https://doi.org/10.31478/201909a
Tweet this! Many individuals who could benefit from buprenorphine – an FDA-approved treatment – are unable to receive it. Authors of this #NAMPerspectives commentary discuss why regulations must keep pace with need: https://doi.org/10.31478/201909a
![]() Tweet this! Educating clinicians on how to treat addiction is imperative in reversing the trends of the opioid crisis and utilizing first-line treatments like buprenorphine: https://doi.org/10.31478/201909a #NAMPerspectives
Tweet this! Educating clinicians on how to treat addiction is imperative in reversing the trends of the opioid crisis and utilizing first-line treatments like buprenorphine: https://doi.org/10.31478/201909a #NAMPerspectives
![]() Tweet this! There is an abundance of stigma that exists around treating people with addiction. A new Commentary outlines how we can combat clinician stigma with addiction treatment education: https://doi.org/10.31478/201909a #NAMPerspectives
Tweet this! There is an abundance of stigma that exists around treating people with addiction. A new Commentary outlines how we can combat clinician stigma with addiction treatment education: https://doi.org/10.31478/201909a #NAMPerspectives
Download the graphics below and share them on social media!


References
- The Underestimated Cost of the Opioid Crisis. November 2017. Executive Office of the President of the United States. Available at: https://www.whitehouse.gov/sites/whitehouse.gov/files/images/The%20Underestimated%20Cost%20of%20the%20Opioid%20Crisis.pdf (accessed September 6, 2019).
- National Survey on Drug Use and Health: Detailed Tables. 2017. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Page 2871.
- Bliley, T. Drug Addiction Treatment Act of 2000. H.R. 2634 Jul 27, 2000. Available at: https://www.congress.gov/bill/106th-congress/house-bill/2634/actions (accessed September 6, 2019).
- National Academies of Sciences, Engineering, and Medicine. 2019. Medications for Opioid Use Disorder Save Lives. Washington, DC: The National Academies Press. https://doi.org/10.17226/25310
- Mattick, R. P., C. Breen, J. Kimber, and M. Davoli. 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Systemic Review. Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002207.pub4/full (accessed September 6, 2019).
- Nasser, A. F., C. Heidbreder, Y. Liu, and P. J. Fudala. 2015. Pharmacokinetics of Sublingual Buprenorphine and Naloxone in Subjects with Mild to Severe Hepatic Impairment (Child-Pugh Classes A, B, and C), in Hepatitis C Virus-Seropositive Subjects, and in Healthy Volunteers. Clinical Pharmacokinetics Aug;54(8):837–49. Available at: https://link.springer.com/article/10.1007%2Fs40262-015-0238-6 (accessed September 6, 2019).
- H. Rept. 106-441 – Drug Addiction Treatment Act of 1999. Sect. Commerce Washington, DC; Nov 3, 1999. Available at: https://www.congress.gov/congressional-report/106th-congress/house-report/441 (accessed on September 4, 2019).
- WESTAT, The Avisa Group. 2006. The SAMHSA Evaluation of the Impact of the DATA Waiver Program. Rockville, MD: Substance Abuse and Mental Health Services Administration; p. 57. Report No.: Task Order 277-00-6111. Available at: https://www.samhsa.gov/sites/default/files/programs_campaigns/medication_assisted/evaluation-impactdata-waiver-program-summary.pdf (accessed on September 6, 2019).
- Dr. Joe Parks’ Testimony at President’s Commission on Combating Drug Addiction and the Opioid Crisis. National Council for Behavioral Health. Available at: https://www.thenationalcouncil.org/press-releases/dr-joe-parks-testimony-presidentscommission-combating-drug-addiction-opioid-crisis (accessed on September 6, 2019).
- Orgera, K., and J. Tolbert. 2019. The Opioid Epidemic and Medicaid’s Role in Facilitating Access to Treatment. Henry J. Kaiser Family Foundation. Available at: https://www.kff .org/medicaid/issue-brief/the-opioid-epidemic-and-medicaidsrole-in-facilitating-access-to-treatment/ (accessed on September 6, 2019).
- Jones, C. M., M. Campopiano, G. Baldwin, and E. McCance-Katz. 2015. National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. American Journal of Public Health June 11;105(8):e55–63. https://doi.org/10.2105/AJPH.2015.302664
- Haff ajee, R. L., L. A. Lin, A. S. B. Bohnert, and J. E. Goldstick. 2019. Characteristics of US Counties With High Opioid Overdose Mortality and Low Capacity to Deliver Medications for Opioid Use Disorder. JAMA Network Open 2(6):e196373–e196373. https://doi.org/10.1001/jamanetworkopen.2019.6373
- Whitehouse, S. Comprehensive Addiction and Recovery Act of 2016. Sect. S.524, 114–198 Jul 22, 2016. Available at: https://www.congress.gov/bill/114th-congress/senate-bill/524/text (accessed on September 6, 2019).
- Huhn, A. S., and K. E. Dunn. 2017. Why aren’t physicians prescribing more buprenorphine? Journal of Substance Abuse Treatment 78:1–7. https://doi.org/10.1016/j.jsat.2017.04.005
- Beletsky, L. 2018. Using Choice Architecture to Integrate Substance Use Services with Primary Care: Commentary on Donohue et al. Journal of Addiction Medicine 12(1):1-3. https://doi.org/10.1097/ADM.0000000000000367
- Lofwall, M. R., and S. L. Walsh. 2014. A Review of Buprenorphine Diversion and Misuse: The Current Evidence Base and Experiences from Around the World. Journal of Addiction Medicine 8(5):315–26. https://doi/org/ 10.1097/ADM.0000000000000045
- Fatseas, M., and M. Auriacombe. 2007. Why buprenorphine is so successful in treating opiate addiction in France. Current Psychiatry Reports 9(5):358–64. https://doi.org/10.1007/s11920-007-0046-2
- Rosenheck, R., and T. Kosten. 2001. Buprenorphine for opiate addiction: potential economic impact. Drug and Alcohol Dependence 1;63(3):253–62. https://doi.org/10.1016/S0376-8716(00)00214-3
- Beletsky, L., S. E. Wakeman, and K. Fiscella. 2019. Practicing What We Preach – Ending Physician Health Program Bans on Opioid-Agonist Therapy. New England Journal of Medicine 381(9):796-797. https://doi.org/10.1056/NEJMp1907875