Clinical Perspectives on Obesity Treatment: Challenges, Gaps, and Promising Opportunities
Introduction
In April 2017, the Roundtable on Obesity Solutions of the National Academies of Sciences, Engineering, and Medicine held a workshop titled The Challenge of Treating Obesity and Overweight with the objective of exploring what is known about current obesity treatment approaches in adults and children and the challenges in implementing them. Presenters described currently available modalities, including behavioral, medical, and surgical approaches. Emerging treatment modalities, including mobile health, devices, and new pharmacologic approaches were also explored.
This discussion paper highlights the challenges, remaining gaps, and promising opportunities in advancing obesity treatment. The authors discuss challenges facing children and adults with obesity, including access to treatment, risks involved with treatment, responsiveness to treatment, and the importance of multidisciplinary care teams. The authors discuss the need for policy changes to support people with obesity, connect the various sectors that affect treatment outcomes, and improve access to care; and the need for a shift in societal perception about risk factors that cause severe obesity. In thinking about future research opportunities, the authors discuss the need for individualized obesity treatments, especially ones that reduce health disparities, and a better understanding of how bariatric surgery affects clinical outcomes and how technology can help address those gaps. In the following sections, the authors discuss the challenges in the treatment of obesity, followed by the challenges in the treatment of severe obesity. The paper concludes with a section on emerging treatments.
The Treatment of Obesity
Adults
The primary modalities used in adult obesity treatment are lifestyle intervention, pharmacotherapy, and bariatric surgery. Numerous studies have demonstrated that intensive lifestyle interventions (ILI), generally delivered in person—individually or in groups—can be effective in inducing clinically meaningful weight loss in many individuals [1].
Although modalities such as ILI have established efficacy for many patients with obesity, access to care remains a problem. Evidence-based guidelines confirm that the most effective lifestyle interventions are a reduced-calorie diet, increased physical activity, and a structured behavioral-change program. These programs include components such as self-monitoring of food intake, physical activity, and other behaviors, and an on-site, high-intensity (at least 14 sessions delivered over six months) intervention delivered in group or individual sessions by a trained interventionist [1]. Supported by extensive evidence, such programs produce an average weight loss of 5–10 percent of initial body weight over six months, with continued maintenance over an additional six months of continued treatment [1]. However, barriers such as cost, time, and treatment availability keep these effective treatments out of reach of many who could benefit from them. Interventions delivered remotely by telephone or electronically lead to less weight loss on average but do have the advantage of being more cost-effective for some patients, as well as the ability to be disseminated throughout difficult-to-reach populations (including those in rural settings, older adults, and people with disabilities). Research to improve the reach and effectiveness of remotely delivered behavioral interventions has the potential to expand access to effective weight management treatment.
Despite initial weight loss for many individuals using current lifestyle modalities, long-term maintenance of lost weight is challenging, with multiple physiological and environmental factors promoting weight regain. Pharmacotherapeutic approaches can both enhance initial weight loss and improve longer-term weight maintenance. Currently, five weight management medications are approved for long-term use, with modest efficacy. Concerns over potential adverse effects and costs limit their access and use. Placebo-subtracted weight loss ranges from 3 to 9 percent of initial body weight, but there is considerable variability in response, with the proportion of patients achieving ≥5 or ≥10 percent weight loss greater than placebo for all approved drugs [2]. The only consistent predictor of later weight loss is initial weight loss within the first three months of treatment; therefore, if the patient has not lost at least 5 percent of initial weight after three months at the full medication dose, it is recommended that the medication be discontinued for lack of efficacy and the patient reevaluated [2]. There is a need to identify more reliable predictors of response, such as behavioral and biological predictors, to improve treatment matching and efficacy. In addition, research to identify new or repurposed efficacious pharmacologic treatments (including combination therapy) with acceptable risks is warranted. Lack of insurance coverage and public policy contribute to the low use of pharmacotherapy.
Children and Adolescents
A 1 percent reduction in 16- and 17-year-olds in the United States with obesity and overweight will reduce the number of adults with obesity by 52,821 in the future and increase lifetime quality-adjusted life years by 47,138 years by 2039 [3]. To achieve this target, emerging consensus indicates an urgent need for effective treatment options alongside community and prevention efforts.
In 2007, the Expert Committee on the Assessment, Prevention and Treatment of Child and Adolescent Overweight and Obesity convened by the American Medical Association in collaboration with the Health Resources and Service Administration and the Centers for Disease Control and Prevention recommended a four-stage approach based on age, weight status, presence of comorbidities, and response to treatment [4]. The first two stages, Stage 1 “prevention plus” and Stage 2 “structured weight management,” are delivered in the primary care office by a health care provider. The support of an allied health care provider, such as a dietitian, is also included in Stage 2 treatment. Stage 3 treatment, or the Weight Management Program, is delivered by a multidisciplinary team. Stage 4 treatment, aimed at youth with severe obesity, includes the use of medications, very-low-calorie diets, and/or weight-loss surgery. Stage 3 and 4 treatment options are most often offered at tertiary care obesity programs at children’s hospital settings.
As outlined above, intensive treatment at a multidisciplinary program is widely accepted as the best nonsurgical option for children with obesity [5]. The 2016 US Preventive Services Task Force (USPSTF) report identifies these types of programs as effective in reducing body mass index (BMI) only when they deliver moderate to high-intensity interventions, defined as more than 25 hours of contact with the child and/or family over six months [5]. However, such programs are resource-intensive and not universally available [6]. The findings of the USPSTF of the benefits of treatment when the intervention is of moderate to high intensity provides a strong and compelling reason for universal coverage for comprehensive, intensive behavioral treatment for obesity in children and adolescents. Yet, poor reimbursement for childhood and adolescent obesity treatment continues to be a significant barrier to universal implementation of these treatments [7]. Advocacy around insurance reimbursement is an important gap that must be addressed before comprehensive behavioral treatment can become available to all. Policies and programs driven by multiple sectors and platforms will be integral to making any progress. Multipronged efforts to educate the public, legislators, and health care providers on weight bias, policies, interventions, and research are necessary steps to improve reimbursement for long-term, sustainable interventions.
In addition to difficulties with insurance coverage, additional costs such as those associated with travel, child care for siblings not engaged in treatment, and missed school and work days to attend frequent visits all present challenges to program participation [8,9,10]. New technologies that replace the need for face-to-face contact and yet still promote lifestyle changes may offer one approach to achieving the level of contact recommended by the USPSTF report while minimizing the burden of participation. The use of web-based interventions, mobile apps, and text messaging has led to promising results in adult populations [11]. Although most studies report satisfaction among participants with technology-based program components, long-term significant decreases in BMI among pediatric populations were not achieved [12]. Increasing sophistication of new technologies that include artificial intelligence and passive monitoring of behaviors (such as activity, caloric intake, mood, and so on) to provide feedback and drive behavior change offer opportunities for further innovation. Incorporating new technologies into treatment options may also present a chance to address disparities in outcomes, since adolescents who are minorities are as likely as or more likely than their peers to own smartphones [13]. However, creating an evidence base for the use of technology in pediatric obesity care faces the challenge of research funding cycles that move at a much slower pace than changes in the technology itself. Solving this mismatch is an important step in helping to improve care for children with obesity.
The Treatment of Severe Obesity
Adults
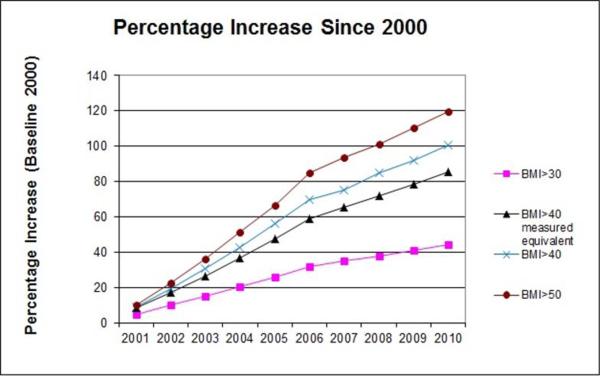
Treating severe obesity (BMI > 120 percent of the 95th percentile or BMI > 40 kg/m2) presents unique challenges to health care providers and communities because of the multidisciplinary approach required across several medical specialties and public health venues. Although the prevalence of obesity overall has leveled off at approximately 35–40 percent of the US population, the subset of this population suffering from severe obesity has continued to increase (see Figure 1) [14,15]. Obesity medicine, a rapidly growing specialty, represents a specialized set of knowledge and skills that focuses on nonsurgical management of patients with obesity.
Figure 1 | Prevalence of Growth of Severe Obesity
SOURCE: Sturm, R., and A. Hattori, International Journal of Obesity, June 2013; 37(6):889-891. Reprinted with permission from Springer Nature.
Treatment Modalities
The complexity required to treat patients with BMI > 40 kg/mm2 is supported by the significant failure rate of dietary interventions, behavioral interventions, and medical therapies, with weight regain occurring even after bariatric surgery. Very-low-calorie diet programs have been shown to be effective in achieving weight loss in severe obesity, but long-term compliance remains a challenge.
Metabolic and bariatric surgery has been considered the gold standard treatment for severe obesity and the most effective option, but there are concerns about long-term efficacy, with data demonstrating that more than 20 percent of patients experience weight regain with recurrence of comorbidities [16,17]. The addition of anti-obesity pharmacotherapy in patients with inadequate weight loss or weight regain after bariatric surgery appears to produce better efficacy. Current evidence suggests that starting medication at a weight plateau may be more effective than waiting for weight regain after bariatric surgery [18].
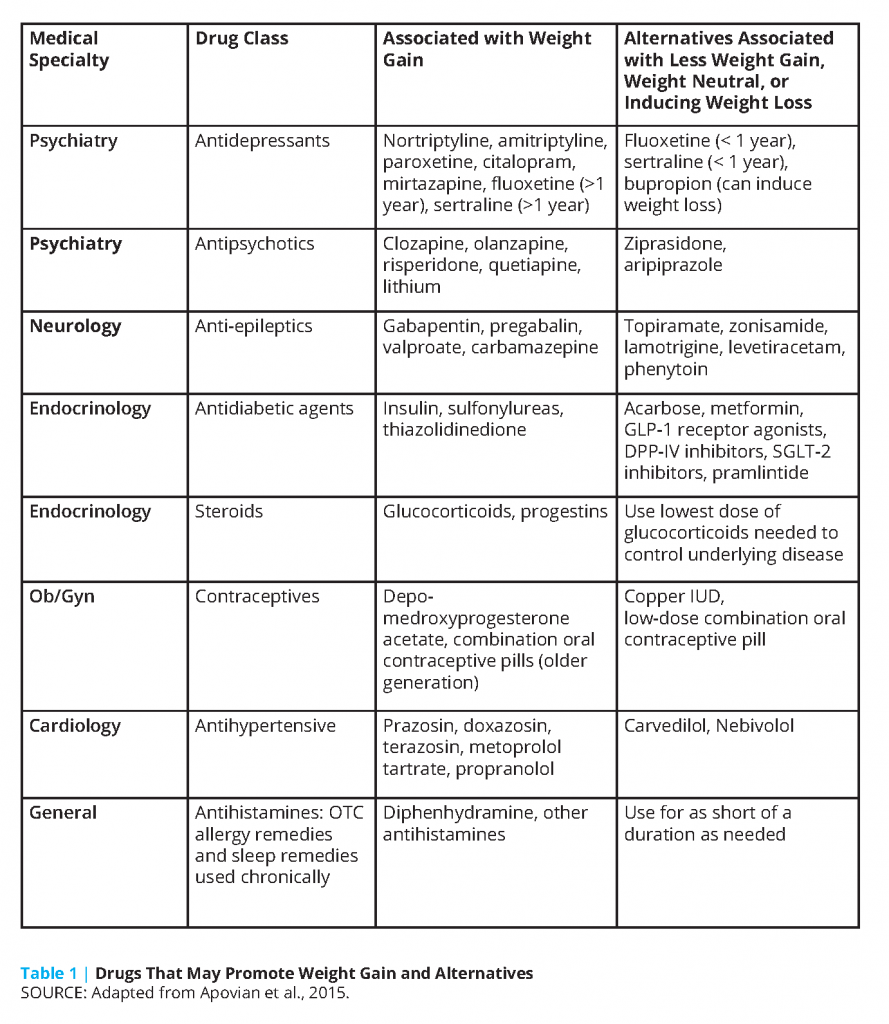
A concept crucial to understanding why failure rates are so high in the treatment of severe obesity is that homeostatic control of body weight by hypothalamic neurons may be damaged in diet-induced obesity [19]. In the disease of obesity, there is a disruption of this homeostasis because of impaired neurohormonal signaling. In cases of severe obesity, it is critical to think of reasons beyond diet that may have affected this set point, such as current or prior medication usage that may have led to weight gain. Several commonly prescribed medicines can contribute to abnormal weight gain or interfere with a patient’s ability to lose weight (see Table 1). These medications include anti-psychotics, anti-depressants, anti-epileptics, insulins and insulin secretagogues, glucocorticoids, progestational hormones and implants, oral contraceptives, beta-blockers, and others [20]. Alternatives to these medicines should be considered and, if possible, changed to those that are weight neutral or to agents that can treat the underlying condition and cause weight loss at the same time. Over-the-counter medicines and supplements should be reviewed for their potential to cause weight gain. Medication lists should be closely evaluated when patients reach plateaus or regain weight after bariatric surgery.
A novel approach to the treatment of severe obesity is incorporating the use of technology. In addition to fitness trackers, web-based programs for self-monitoring, and mobile apps, the use of technology via telemedicine and remote monitoring of patients is becoming more common. The use of Wi-Fi scales, blood pressure cuffs, and glucometers allows patient data to be transmitted to a health care provider. A patient can receive feedback even when not attending an office visit, which may improve long-term adherence to their weight management plan. Some centers use telemedicine to administer a weight management program and provide a more intensive behavioral intervention [20].
Another substantial barrier to providing effective care to patients with severe obesity is lack of insurance coverage. Although patient visits may be covered if comorbid conditions are present, medications often are not covered. In 2010, the Affordable Care Act extended coverage by private and public insurers for behavior modification for obesity and for bariatric surgery. Approximately 50 percent of employers who provide health insurance opt in for anti-obesity medication coverage [21]. In a study published by Gomez and Stanford in 2017, Medicare did not provide coverage of anti-obesity medicine, and eight out of 34 states examined provided some type of coverage. Coverage has expanded slightly since this publication [22].
Bariatric Surgery
Bariatric surgery is the most effective modality for weight loss and maintenance in patients with severe obesity, but for a number of reasons, including costs, limited access to care, and patient concerns about adverse events, use is limited to a small fraction of those who are eligible for the procedure. Although recent studies have confirmed that bariatric surgical procedures can have beneficial effects for many obesity-related comorbid conditions, particularly type 2 diabetes, few studies have evaluated the long-term benefits and adverse effects of vertical sleeve gastrectomy, which is currently the most commonly performed bariatric surgical procedure. There are also limited data on safety and efficacy in racial and ethnic minority populations.
The overall goal of bariatric surgery is weight loss and comorbid disease remission or improvement for a patient with severe obesity, as defined by the BMI and related comorbid conditions. Comorbid conditions, as well as functional impairments associated with moderate to severe obesity, are highly variable. In addition, the weight loss response to standardized intervention, including lifestyle intervention and bariatric surgical procedures, is highly variable [23,24]. A personalized medicine approach would greatly improve the selection of patients from the standpoint of risk, as well as efficacy, if the factors involved in risks and the variable outcomes could be clearly identified.
Safety. The short-term (30-day) risks of specific bariatric surgical procedures are relatively well defined by reports from the Longitudinal Assessment of Bariatric Surgery (LABS) consortium as well as the Registry of the American College of Surgeons/American Society for Metabolic and Bariatric Surgery accreditation program [25,26]. Longer-term risks or complications are considerably more difficult to quantify because these bariatric surgical procedures are performed at experienced regional centers to maximize safety. When complications occur, however, the patients commonly seek care in their local medical environment. The necessity for re-operations or revisions may or may not lead the patient to return to the original bariatric surgical center. Revisions may be performed on patients who have lost less than the desirable weight or experienced undesirable weight regain. Conversion to a procedure associated with greater weight loss is one example of such revision. Revisions may also be done for complications. One such example is conversion of a patient who is undergoing sleeve gastrectomy to Roux-en-Y gastric bypass (RYGB) for the development of severe gastroesophageal reflux disease [27]. Reversals have been considerably less common, particularly after RYGB. Sleeve gastrectomy is not reversible. Laparoscopic adjustable gastric banding (LAGB) has been widely perceived as a reversible procedure [28], although this was not the case in the LABS consortium—at Year 7 of the study, 22 percent of LAGB had been removed [24]. Revisions may also be done for metabolic complications such as micronutrient deficiency secondary to diminished intake, vomiting, or malabsorption. Problematic recurrent hypoglycemia, although rare, may also require reversal. Alcohol use disorder has been identified as a complication of gastric bypass [29]. The frequency and etiology of this phenomenon requires further definition.
Weight Loss. As reported by LABS Consortium at Year 7, the weight loss after LAGB and RYGB was highly variable and not predictable by usual clinical characteristics before operation [24]. The institution or addition of lifestyle intervention as well as pharmacotherapy to patients desiring additional weight loss beyond that maintained by their bariatric surgical procedure is a viable intervention that requires further research.
Overall, more research is needed to determine how much weight loss is needed to accomplish a specific clinical outcome in a specific patient using a specific bariatric surgical intervention. For example, gastric bypass has been documented to induce diabetes remission [30], although this effect is not uniform among all surgical candidates—to be able to predict such a response for an individual patient is the fundamental goal of precision medicine and the next clinical target to be embraced by the bariatric surgical community. With the efficacy and safety profiles of common bariatric surgical interventions clearly established, the next step is to enhance prediction of specific clinical implications based on a patient’s comorbidity profile and baseline clinical characteristics.
Policy Implications. The application of bariatric surgery to patients who meet criteria for such surgery remains as low as 2 percent or less per year in the United States [31]. More precise data are needed to identify the explanation(s) for this low application of bariatric surgery. Factors may include lack of knowledge about the specific benefits, fear of complications, discouragement from family or health care providers, and/or lack of social support [32]. In addition, knowledge of the progress that has been made in achieving safe and efficacious outcomes in bariatric surgery is not widely known by the nonsurgical medical community.
Lack of insurance coverage appears to be a secondary explanation for the low utilization of bariatric surgery [33]. Insurance providers commonly insist on higher levels of evidence to support bariatric surgery in specific populations than is required for other covered surgical procedures. Additionally, high insurance co-pays for prospective bariatric surgical candidates as well as low physician reimbursement rates, as is the case for Medicaid-covered patients, play a role in explaining the low utilization of bariatric surgery, although data clarifying these important issues are lacking.
An overall goal of additional research is to enable personalized medicine to be applied to weight loss and obesity treatment generally, and bariatric surgery specifically. If sufficient research can be applied to enable increased personalization of this care, it is reasonable to predict that the application of bariatric surgery will increase.
Children and Adolescents
One of the significant challenges faced by children with severe obesity is limited access to appropriate care and resources. This is keenly experienced in low-income and minority populations, who have both increased prevalence and severity of obesity, even at a young age. In a recent study, young children aged 2–5 years with severe obesity had a higher odds ratio (OR) of being racial/ethnic minorities, members of households with lower educational attainment (OR 2.4, 95 percent CI [1.4, 4.0]), in single-parent-headed households (OR 2.0, 95 percent CI [1.3, 3.0]), and in poverty (OR 2.1, 95 percent CI [1.1, 4.0]) [34].
Although the vast majority of children have access to a primary care provider, primary care-based interventions have not been shown to provide effective weight loss, especially for children with severe obesity. One intervention that has shown success in primary care is the Brief Motivational Interviewing to Reduce Child BMI (BMI2) trial, which used motivational interviewing delivered by primary care providers and dietitians to treat children ages 3 to 8 years with excess weight. However, eligibility for the intervention required a body mass index between the 85th and the 97th percentile, thus excluding some children with severe obesity [35].
The application of metabolic and bariatric surgery as a safe and effective treatment strategy for severe childhood obesity has been the focus of a growing body of literature over the past two decades, and recent data from ongoing prospective multi-institutional cohorts have provided important information to the medical community and lent additional strength to this therapeutic paradigm. In addition to providing robust and uniform data, recent studies have also served to highlight a number of evidence gaps that merit further investigation as well as provide insights related to disparities in access to bariatric surgical care.
Safety and Efficacy. Teen-LABS is a prospective observational National Institutes of Health-funded study of 242 adolescents (<19 years of age; mean age 17.1 years) who underwent RYGB (n=161), vertical sleeve gastrectomy (n=67), or adjustable gastric band (n=14) at one of five US centers [36]. In addition to being the largest ongoing investigation of adolescent bariatric surgical outcomes, designed to evaluate general safety and efficacy measures as well as provide assessment of long-term health effects after surgical weight loss, this study has served to draw attention to the general health status of adolescents with severe obesity who present for such intervention [24,36]. In addition to reporting the baseline prevalence of numerous obesity-related comorbid conditions such as dyslipidemia (74.4 percent), obstructive sleep apnea (56.6 percent), joint pain (46.6 percent), hypertension (45 percent), fatty liver disease (36.9 percent), chronic kidney disease (19.2 percent), and diabetes mellitus (13.6 percent), investigators noted the overall multiplicity of comorbid disease within the cohort, whereby 51 percent of participants demonstrated four or more major comorbid conditions before undergoing bariatric surgery [36].
In addition to the higher-than-anticipated rates of related disease burden, initial reports from the Teen-LABS study consortium have identified clinical and demographic variables that serve as independent predictors of baseline cardio-metabolic disease risk factors. Namely, higher BMI and male sex increase the relative risk of several known cardiovascular disease risk factors [24]. Furthermore, initially favorable short-term results and complication profiles have been bolstered by reports of midterm (three-year post-op) longitudinal analyses within this same cohort. In a related analysis by Inge et al., investigators showed a mean reduction in weight of 27 percent with significant rates of remission of baseline type 2 diabetes (95 percent), abnormal kidney function (86 percent), elevated blood pressure (74 percent), and dyslipidemia (66 percent) [25]. Corresponding analysis of changes in specific cardiovascular disease risk factors by Michalsky et al. [26] have concluded that increased weight loss after bariatric surgical intervention, female sex, and younger age at the time of surgery predict a higher probability of resolution of specific risk factors (i.e., elevated blood pressure, dyslipidemia, and abnormally elevated levels of high-sensitivity C reactive protein, a marker of systemic inflammation) [26]. Collectively, these recent findings may lead to further refinement in patient selection criteria and recommendations for optimal timing of adolescent bariatric surgery even within the age group itself.
Although Teen-LABS and other recent reports have provided extremely valuable information that has helped to inform the medical community about the overall risks and benefits of bariatric surgery in the pediatric population, the recent reporting of longer-term data (five years and beyond) serves an equally important role in helping to define and broaden our understanding of the potential health effects of bariatric surgery within the context of this population. Olbers et al. [37], in a comparative analysis of adolescents undergoing RYGB versus matched controls made up of (1) adolescents undergoing lifestyle intervention only and (2) adult participants undergoing RYGB, concluded that the observed change in weight (−36.8 kg), BMI (−13.1 kg/m2), and improvements in cardiovascular disease risk factors at five years were similar to corresponding outcomes in adults while simultaneously distinguishing themselves from the nonsurgical adolescent controls who experienced a rise in BMI over the same study period (mean +3.3 kg/m2).
However, despite favorable outcomes, results of this analysis also served to highlight post-operative nutritional deficiencies similar to previous reports, emphasizing the need to provide close long-term follow-up [25]. Additional prospective data from the Follow-up of Adolescent Bariatric Surgery study, examining outcomes among 58 adolescents undergoing RYGB with a mean follow-up of 8.0 years (range 5.4–12.5 years) provides the most robust long-term analysis to date [27]. In addition to demonstrating a durable change in excess weight (−29.2 kg/m2) investigators showed significant reduction in the prevalence of key cardiovascular disease risk factors when compared with baseline (pre-bariatric surgery), including elevated blood pressure (16 percent versus 47 percent), dyslipidemia (38 percent versus 86 percent), and type 2 diabetes (2 percent versus 16 percent). Although it is anticipated that these ongoing studies will yield additional long-term data and provide important insights in the future, a number of related opportunities remain ripe for further investigation, including the determination of optimal lower age limit, the potential effect on bone density, long-term musculoskeletal and cardio-metabolic health, quality of life measures, fertility, and epigenetics.
Utilization. Despite increasing evidence supporting the utilization of metabolic and bariatric surgery in the treatment of severe childhood obesity, the procedural prevalence of weight loss operations among adolescents has remained relatively low compared with the affected adult population. Although a rise in procedural prevalence was reported in the early twenty-first century, current estimates in the United States remain relatively small: between 1,000 and 1,600 cases per year [29,38]. Although multiple variables are no doubt responsible for the relative paucity of adolescent bariatric operations, several factors, including attitudes and related referral patterns among primary care providers, medical subspecialists, and surgeons alike are probable contributors. Recent results of a national random sample of pediatricians and family practitioners in the United States showed that nearly half (48 percent) of respondents said they would never consider referring an adolescent for weight loss surgery [32], and in the United Kingdom, surgical subspecialists appeared to demonstrate a higher degree of reluctance compared with nonsurgical respondents [33].
Barriers. In addition to the need to address related professional education, including the ongoing development of best practice guidelines designed to guide referral practices to tertiary care facilities capable of providing multidisciplinary pediatric-specific bariatric care, evidence about limitations in insurance authorization also require consideration. A recent review of 57 adolescents with clinical indications for weight loss surgery at one of five centers (2009 to 2011) with defined bariatric insurance benefits showed that only 47 percent received initial coverage authorization [23]. Although 80 percent of primary insurance denials were ultimately overturned after multiple appeals (as many as five), 11 percent of surgical candidates never obtained authorization. Age less than 18 years and specific procedure type were cited as the most common reasons for denial. Collectively, these reports not only highlight the ongoing challenges faced by the pediatric population with severe obesity, but highlight the need for continuous efforts focused on medical education, public health policy, and patient advocacy designed to improve overall access to care.
Emerging Treatments
Pharmacologic
Many pharmacologic targets have been evaluated for managing excess adiposity. In the past, the success rate in developing safe and effective medicines has not been very high [39]. Newer medications recently introduced or still in development tend to be more selective for known weight control targets and hence are not only effective, but safe to administer across a wide group of adults varying in age and BMI. Specific recent developments are reviewed in the following sections.
The core physiological derangements leading to excess adiposity involve disturbances in energy intake and expenditure. There are 11 rare nonsyndromic monogenic forms of human obesity for which the underlying mutations are known. A therapeutic approach is now available for treating one of these inherited forms of obesity, deficiency in the leptin receptor [40].
Another pathway involves mutations in the melanocortin-4 (MC4) receptor gene that are accompanied by early onset obesity. The MC4 receptor is a key component of a pathway that controls appetite, satiety, and energy homeostasis. A recently introduced MC4 receptor peptide agonist, setmelanotide [41], is being developed for six monogenic MC4 deficiency states: pro-opiomelanocortin (POMC) deficiency obesity, leptin receptor deficiency obesity, Bardet-Biedl syndrome, Alström syndrome, POMC heterozygous deficiency obesity, and POMC epigenetic disorders [42]. Advances in peptide therapeutics promise to open new opportunities for managing body weight in adults with obesity. Long-acting parenteral and oral GLP-1 agonists are entering late-stage clinical trials [43,44]. More than 23 new peptides are in development [20]. Combining two peptides with complementary modes of action is another area in development [44].
The SGLT2 inhibitor class of drugs used to treat diabetes also promotes weight loss and reduces cardiovascular and renal events [45]. An important question is if similar event reductions are observed in patients with obesity but who do not have type 2 diabetes. Combinations of orally ingested medications using currently approved drugs are entering late-stage clinical trials [46].
Medicines and combination drugs that target hedonic mechanisms are being evaluated for their weight loss efficacy. A medication that blocks the μ opioid receptor in the arcuate nucleus of the brain showed promising effects on hedonic pathways in an early-phase study [47].
Novel approaches, including “omics” platforms, are also advancing target discovery and identifying safety concerns [48].
Devices
A wide array of recently developed medical devices with different mechanisms of action are now approved by the Food and Drug Administration (FDA) for short-term weight control [49,50]. These devices can be classified into four types: gastric bands that restrict food intake, electrical systems that stimulate the Vagus nerve and inhibit food intake, space-occupying gastric balloon systems, and gastric emptying systems that allow for draining stomach contents before gastrointestinal absorption. All of these systems have modest efficacy with treatment responses in the range of the higher-efficacy FDA-approved drugs for weight loss. However, the adverse effects that accompany these costly devices vary but can be serious.
Lifestyle Measures
The cornerstone of obesity management is lifestyle management. Many new lifestyle treatments are being introduced and critically evaluated in clinical trials. These include improving the duration and quality of sleep; promoting greater levels of physical activity, including with devices such as standing desks; and using acceptance-based and cognitive behavioral therapies. All of these approaches can be used as part of internet-based weight control programs that include features such as food photography, step-counting, and rapid therapist feedback [51,52,53].
Challenges
Although effective and safe pharmacologic, bariatric surgical, and lifestyle therapies are now available and new ones are on the horizon, several challenges persist that limit their widespread implementation. First, the concept of obesity as a “disease” remains controversial, including among many health care workers. People with obesity are often viewed as lacking willpower or self-control and as having psychological problems that limit their ability to restrict food intake. A prevailing view is that simply eating less and exercising more will transform the person with obesity into a healthy person whose weight is normal. These misconceptions, which fail to recognize modern concepts in the regulation of energy balance and body weight, place barriers for care at many levels. Bias and fat-shaming create an atmosphere in health care that is not conducive to effective and compassionate care.
Once the motivated person with obesity seeks care, expert facilities may not be available in their community. Most physicians in primary care are ill-equipped to deliver the established high-intensity lifestyle treatments that can lead to lasting weight loss and improved health [1]. This lack of high-quality treatment programs is particularly notable in remote settings. Ironically, remote settings are often the regions of the United States that have the highest prevalence rates of obesity and diabetes. An important development is the training of physicians and other health care workers specifically in the area of weight management so that this lack of expert care in some communities may eventually be alleviated. Organizations such as the YMCA (https://www.ymcatriangle.org/programs-services/health-and-wellness/diabetes-prevention-program-0) are also currently offering programs that might fill some community gaps in obesity management.
Although several medicines for the treatment of obesity are now FDA approved and new ones are in development, a challenge is creating drugs that are both highly effective and have a good safety threshold. The drugs now available lead to weight losses in the range of about 3–9 percent above placebo at one year [39]. This treatment efficacy falls within the established paradigm. However, to maintain drug-induced weight loss at present requires a lifelong commitment to therapy. Cost, side effects, and the desire to lose even greater amounts of weight makes such adherence difficult for many patients, and treatment recidivism rates are relatively high. These observations place an even greater burden on emerging drug therapies that ideally will achieve larger relative amounts of weight loss but have minimal side effects.
As high-intensity lifestyle and high-efficacy weight loss treatments face the aforementioned challenges to their adoption, bariatric surgery is an increasingly attractive option for many people with severe obesity. Bariatric surgery is increasingly being evaluated for use in adults whose BMIs fall within a large fraction of the US population (i.e., >30 kg/m2). How, as a research community, do we establish when traditional measures such as lifestyle modification give way to more aggressive surgical treatments? What kinds of risk-benefit studies are needed to answer these kinds of questions? These challenging discussion topics are on the immediate horizon as the obesity epidemic continues to advance across the United States and other nations.
Overarching Themes
There are effective treatment approaches for childhood and adult obesity, but these treatments are not accessible to everyone, some have risks, and not all treatments are appropriate for all patients. Furthermore, patients with severe obesity require multidisciplinary teams that may not be accessible to all patients.
Establishing and sustaining effective treatment can happen only if federal and local public policy makers understand the pathophysiology of obesity and recognize the physical and emotional needs of this distinct population of children and adults. A shift in public perception of the breadth of the risk factors that cause severe obesity will have to be integrated into any public policy effort. Most importantly, the idea that personal responsibility plays the predominant role in how and why an individual develops severe obesity must be challenged vociferously. Only in reversing this preconception can we create the right environment for a cogent public policy and population health platform that supports access to and coverage of existing and emerging treatment options for severe obesity.
Despite the existence of modestly effective treatments, major identified gaps in comprehensive and effective care include:
- a need for improved tailoring of interventions based on research to better match the treatment to an individual patient;
- a need for more treatments with improved efficacy and safety profiles and efforts to reduce health disparities, including additional research on treatment responses in diverse populations;
- facilitation of connections between sectors that affect treatment outcomes (e.g., clinical providers such as physicians and dietitians, community entities such as schools, financial institutions such as insurers);
- improved access to care for adults and children with obesity; and
- additional efforts to understand the ways in which technology can help address these gaps.
A significant gap in the field of metabolic and bariatric surgery is the inability to determine how much weight loss is required to achieve a specific clinical response in regard to comorbid conditions, cancer risk, and mortality. Weight loss has been associated with decreasing cancer risk [54], but specificity remains an issue; for patients with variable risk factors in addition to obesity, understanding the interplay of co-occurring conditions should be a research priority. Similar to cancer, understanding the role of bariatric surgery in inducing diabetes remission is yet another presently underdeveloped subfield. The integration of personalized medicine in obesity research, and more specifically bariatric surgery, to predict specific outcomes in specific patient populations is invariably the next chapter in this evolving field.
Join the conversation!
![]() Tweet this! Effective treatment for severe obesity exists, but it’s not available to everyone who needs it. The future of obesity treatment must focus on health disparities: https://doi.org/10.31478/201809b #NAMPerspectives
Tweet this! Effective treatment for severe obesity exists, but it’s not available to everyone who needs it. The future of obesity treatment must focus on health disparities: https://doi.org/10.31478/201809b #NAMPerspectives
![]() Tweet this! Adults and children differ emotionally, physically, and mentally. Obesity treatments for the two groups must consider all of these differences: https://doi.org/10.31478/201809b #NAMPerspectives
Tweet this! Adults and children differ emotionally, physically, and mentally. Obesity treatments for the two groups must consider all of these differences: https://doi.org/10.31478/201809b #NAMPerspectives
![]() Tweet this! Obesity treatment is complex & must consider all aspects of a patient’s life, including medications that might be causing weight gain. This #NAMPerspectives discussion paper advocates for a comprehensive view of future treatment: https://doi.org/10.31478/201809b
Tweet this! Obesity treatment is complex & must consider all aspects of a patient’s life, including medications that might be causing weight gain. This #NAMPerspectives discussion paper advocates for a comprehensive view of future treatment: https://doi.org/10.31478/201809b
![]() Tweet this! Our newest #NAMPerspectives paper examines a range of treatments for obesity, including bariatric surgery, pharmacologic interventions, medical devices, and lifestyle changes: https://doi.org/10.31478/201809b
Tweet this! Our newest #NAMPerspectives paper examines a range of treatments for obesity, including bariatric surgery, pharmacologic interventions, medical devices, and lifestyle changes: https://doi.org/10.31478/201809b
Download the graphics below and share them on social media!
References
- Jensen, M. D., D. H. Ryan, C. M. Apovian, J. D. Ard, A. G. Comuzzie, K. A. Donato, F. B. Hu, V. S. Hubbard, J. M. Jakicic, R. F. Kushner, C. M. Loria, B. E. Millen, C. A. Nonas, F. X. Pi-Sunyer, J. Stevens, V. J. Stevens, T. A. Wadden, B. M. Wolfe, S. Z. Yanovski, H. S. Jordan, K. A. Kendall, L. J. Lux, R. Mentor-Marcel, L. C. Morgan, M. G. Trisolini, J. Wnek, J. L. Anderson, J. L. Halperin, N. M. Albert, B. Bozkurt, R. G. Brindis, L. H. Curtis, D. DeMets, J. S. Hochman, R. J. Kovacs, E. M. Ohman, S. J. Pressler, F. W. Sellke, W. K. Shen, S. C. Smith Jr., G. F. Tomaselli, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the Obesity Society. 2014. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Obesity Society. Circulation 129(25 Suppl 2):S102-138. https://doi.org/10.1161/01.cir.0000437739.71477.ee
- Yanovski, S. Z., and J. A. Yanovski. Long-term drug treatment for obesity: A systematic and clinical review. 2014. JAMA 311(1):74-86. https://doi.org/10.1001/jama.2013.281361
- Wang, L. Y., M. Denniston, S. Lee, D. Galuska, and R. Lowry. 2010. Long-term health and economic impact of preventing and reducing overweight and obesity in adolescence. Journal of Adolescent Health 46(5):467-473. https://doi.org/10.1016/j.jadohealth.2009.11.204
- Barlow, S. E., and Expert Committee. 2007. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 120 Suppl 4:S164-192. https://doi.org/10.1542/peds.2007-2329C
- Whitlock, E. P., E. A. O’Connor, S. B. Williams, T. L. Beil, and K. W. Lutz. 2010. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics 125(2):e396-418. https://doi.org/10.1542/peds.2009-1955
- US Preventive Services Task Force (USPSTF), D. C. Grossman, K. Bibbins-Domingo, S. J. Curry, M. J. Barry, K. W. Davidson, C. A. Doubeni, J. W. Epling Jr., A. R. Kemper, A. H. Krist, A. E. Kurth, C. S. Landefeld, C. M. Mangione, M. G. Phipps, M. Silverstein, M. A. Simon, and C. W. Tseng. 2017. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 317(23):2417-2426. https://doi.org/10.1001/jama.2017.6803
- Slusser, W., K. Staten, K. Stephens, L. Liu, C. Yeh, S. Armstrong, D. A. DeUgarte, and M. Haemer. 2011. Payment for obesity services: Examples and recommendations for stage 3 comprehensive multidisciplinary intervention programs for children and adolescents. Pediatrics 128 Suppl 2:S78-85. Available at: https://scholars.duke.edu/display/pub758079 (accessed September 1, 2020).
- Arai, L., M. Panca, S. Morris, K. Curtis-Tyler, P. J. Lucas, and H. M. Roberts. 2015. Time, monetary and other costs of participation in family-based child weight management interventions: Qualitative and systematic review evidence. PLoS One 10(4):e0123782. https://doi.org/10.1371/journal.pone.0123782
- Sallinen, B. J., S. Schaffer, and S. J. Woolford. 2013. In their own words: Learning from families attending a multidisciplinary pediatric weight management program at the YMCA. Childhood Obesity 9(3):200-207. https://doi.org/10.1089/chi.2012.0106
- Woolford, S. J., B. J. Sallinen, S. Schaffer, and S. J. Clark. 2012. Eat, play, love: Adolescent and parent perceptions of the components of a multidisciplinary weight management program. Clinical Pediatrics 51(7):678-684. https://doi.org/10.1177/0009922812440839
- Hutchesson, M. J., M. E. Rollo, R. Krukowski, L. Ells, J. Harvey, P. J. Morgan, R. Callister, R. Plotnikoff, and C. E. Collins. 2015. Ehealth interventions for the prevention and treatment of overweight and obesity in adults: A systematic review with meta-analysis. Obesity Reviews 16(5):376-392. https://doi.org/10.1111/obr.12268
- Turner, T., D. Spruijt-Metz, C. K. Wen, and M. D. Hingle. 2015. Prevention and treatment of pediatric obesity using mobile and wireless technologies: A systematic review. Pediatric Obesity 10(6):403-409. https://doi.org/10.1111/ijpo.12002
- Lenhart, A. 2015. Teens, social media & technology overview 2015. Pew Research Center. Available at: http://www.pewinternet.org/2015/04/09/teens-social-media-technology-2015 (accessed February 18, 2018).
- Centers for Disease Control and Prevention (CDC). 2017. Adult Obesity Prevalence Maps. Available at: https://www.cdc.gov/obesity/data/prevalence-maps.html (accessed January 11, 2018).
- Sturm, R., and A. Hattori. 2013. Morbid obesity rates continue to rise rapidly in the United States. International Journal of Obesity (2005) 37(6):889-891. https://doi.org/10.1038/ijo.2012.159
- Magro, D. O., B. Geloneze, R. Delfini, B. C. Pareja, F. Callejas, and J. C. Pareja. 2008. Long-term weight regain after gastric bypass: A 5-year prospective study. Obesity Surgery 18(6):648-651. https://doi.org/10.1007/s11695-007-9265-1
- Sumithran, P., L. A. Prendergast, E. Delbridge, K. Purcell, A. Shulkes, A. Kriketos, and J. Proietto. 2011. Long-term persistence of hormonal adaptations to weight loss. New England Journal of Medicine 365(17):1597-1604. https://doi.org/10.1056/NEJMoa1105816
- Stanford, F. C., N. Alfaris, G. Gomez, E. T. Ricks, A. P. Shukla, K. E. Corey, J. S. Pratt, A. Pomp, F. Rubino, and L. J. Aronne. 2017. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surgery for Obesity and Related Diseases 13(3):491-500. Available at: https://scholar.harvard.edu/fatimacodystanford/publications/utility-weight-loss-medications-after-bariatric-surgery-weight-0 (accessed September 1, 2020).
- Kumar, R. B., and L. J. Aronne. 2014. Hypothalamic inflammation: Is there evidence for human obesity? Current Obesity Reports 3(2):242-247. https://doi.org/10.1007/s13679-014-0104-0
- Apovian, C. M., L. A. Aronne, and A. G. Powell. 2015. Clinical management of obesity, 1st, edited by M. Beasley and V. Runowicz. West Islip, NY: Professional Communications, Inc.
- National Academies of Sciences, Engineering, and Medicine. 2017. The Challenge of Treating Obesity and Overweight: Proceedings of a Workshop. Washington, DC: The National Academies Press. https://doi.org/10.17226/24855
- Gomez, G., and F. C. Stanford. 2018. US health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. International Journal of Obesity 42(3):495-500. https://doi.org/10.1038/ijo.2017.287
- Inge, T. H., T. W. Boyce, M. Lee, L. Kollar, T. M. Jenkins, M. L. Brandt, M. Helmrath, S. A. Xanthakos, M. H. Zeller, C. M. Harmon, A. Courcoulas, and M. P. Michalsky. 2014. Access to care for adolescents seeking weight loss surgery. Obesity 22(12):2593-2597. Available at: https://onlinelibrary.wiley.com/doi/pdf/10.1002/oby.20898 (accessed September 1, 2020).
- Michalsky, M. P., T. H. Inge, M. Simmons, T. M. Jenkins, R. Buncher, M. Helmrath, M. L. Brandt, C. M. Harmon, A. Courcoulas, M. Chen, M. Horlick, S. R. Daniels, E. M. Urbina, and Teen Labs Consortium. 2015. Cardiovascular risk factors in severely obese adolescents: The teen longitudinal assessment of bariatric surgery (Teen-LABS) study. JAMA Pediatrics 169(5):438-444. https://doi.org/10.1001/jamapediatrics.2014.3690
- Inge, T. H., A. P. Courcoulas, T. M. Jenkins, M. P. Michalsky, M. A. Helmrath, M. L. Brandt, C. M. Harmon, M. H. Zeller, M. K. Chen, S. A. Xanthakos, M. Horlick, and C. R. Buncher. 2016. Weight loss and health status 3 years after bariatric surgery in adolescents. New England Journal of Medicine 374(2): 113-123. https://doi.org/10.1056/NEJMoa1506699
- Michalsky, M. P., T. H. Inge, T. M. Jenkins, C. Xie, A. Courcoulas, M. Helmrath, M. L. Brandt, C. M. Harmon, M. Chen, J. B. Dixon, E. M. Urbina, and Teen Labs Consortium. 2018. Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics 141(2):e20172485. https://doi.org/10.1542/peds.2017-2485
- Inge, T. H., T. M. Jenkins, S. A. Xanthakos, J. B. Dixon, S. R. Daniels, M. H. Zeller, and M. A. Helmrath. 2017. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): A prospective follow-up analysis. The Lancet Diabetes & Endocrinology 5(3):165-173. https://doi.org/10.1016/S2213-8587(16)30315-1
- Chapman A. E., G. Kiroff, P. Game, B. Foster, P. O’Brien, J. Ham, and G. J. Maddern. 2004. Laparoscopic adjustable gastric banding in the treatment of obesity: A systematic literature review. Surgery 135(3):326-351. https://doi.org/10.1016/S0039-6060(03)00392-1
- Zwintscher, N. P., K. S. Azarow, J. D. Horton, C. R. Newton, and M. J. Martin. 2013. The increasing incidence of adolescent bariatric surgery. Journal of Pediatric Surgery 48(12):2401-2407. https://doi.org/10.1016/j.jpedsurg.2013.08.015
- Hall, T. C., M. G. Pellen, P. C. Sedman, and P. K. Jain. 2010. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obesity Surgery 20(9):1245-1250. https://doi.org/10.1007/s11695-010-0198-8
- Kelleher, D. C., C. T. Merrill, L. T. Cottrell, E. P. Nadler, and R. S. Burd. 2013. Recent national trends in the use of adolescent inpatient bariatric surgery: 2000 through 2009. JAMA Pediatrics 167(2):126-132. https://doi.org/10.1001/2013.jamapediatrics.286
- Woolford, S. J., S. J. Clark, A. Gebremariam, M. M. Davis, and G. L. Freed. 2010. To cut or not to cut: Physicians’ perspectives on referring adolescents for bariatric surgery. Obesity Surgery 20(7):937-942. https://doi.org/10.1007/s11695-010-0152-9
- Penna, M., S. Markar, J. Hewes, A. Fiennes, N. Jones, and M. Hashemi. 2014. Adolescent bariatric surgery: Thoughts and perspectives from the UK. International Journal of Environmental Research and Public Health 11(1):573-582. https://doi.org/10.3390/ijerph110100573
- Tester, J. M., T. T. Phan, J. M. Tucker, C. W. Leung, M. L. Dreyer Gillette, B. R. Sweeney, S. Kirk, A. Tindall, S. E. Olivo-Marston, and I. U. Eneli. 2018. Characteristics of children 2 to 5 years of age with severe obesity. Pediatrics 141(3):e20173228. https://doi.org/10.1542/peds.2017-3228
- Resnicow, K., F. McMaster, A. Bocian, D. Harris, Y. Zhou, L. Snetselaar, R. Schwartz, E. Myers, J. Gotlieb, J. Foster, D. Hollinger, K. Smith, S. Woolford, D. Mueller, and R. C. Wasserman. 2015. Motivational interviewing and dietary counseling for obesity in primary care: An RCT. Pediatrics 135(4):649-657. https://doi.org/10.1542/peds.2014-1880
- Inge, T. H., M. H. Zeller, T. M. Jenkins, M. Helmrath, M. L. Brandt, M. P. Michalsky, C. M. Harmon, A. Courcoulas, M. Horlick, S. A. Xanthakos, L. Dolan, M. Mitsnefes, S. J. Barnett, R. Buncher, and Teen Labs Consortium. 2014. Perioperative outcomes of adolescents undergoing bariatric surgery: The teen-longitudinal assessment of bariatric surgery (Teen-LABS) study. JAMA Pediatrics 168(1):47-53. https://doi.org/10.1001/jamapediatrics.2013.4296
- Olbers, T., A. J. Beamish, E. Gronowitz, C. E. Flodmark, J. Dahlgren, G. Bruze, K. Ekbom, P. Friberg, G. Gothberg, K. Jarvholm, J. Karlsson, S. Marild, M. Neovius, M. Peltonen, and C. Marcus. 2017. Laparoscopic roux-en-y gastric bypass in adolescents with severe obesity (AMOS): A prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol 5(3):174-183. https://doi.org/10.1016/S2213-8587(16)30424-7
- Kelleher, D. C., C. T. Merrill, L. T. Cottrell, E. P. Nadler, and R. S. Burd. 2012. Recent national trends in the use of adolescent inpatient bariatric surgery: 2000 through 2009. JAMA Pediatrics 167(2):126-132. https://doi.org/10.1001/2013.jamapediatrics.286
- Heymsfield, S. B., and T. A. Wadden. 2017. Mechanisms, pathophysiology, and management of obesity. New England Journal of Medicine 376(3):254-266. https://doi.org/10.1056/NEJMra1514009
- Heymsfield, S. B., A. S. Greenberg, K. Fujioka, R. M. Dixon, R. Kushner, T. Hunt, J. A. Lubina, J. Patane, B. Self, P. Hunt, and M. McCamish. 1999. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. JAMA 282(16):1568-1575. https://doi.org/10.1001/jama.282.16.1568
- Collet, T. H., B. Dubern, J. Mokrosinski, H. Connors, J. M. Keogh, E. Mendes de Oliveira, E. Henning, C. Poitou-Bernert, J. M. Oppert, P. Tounian, F. Marchelli, R. Alili, J. Le Beyec, D. Pepin, J. M. Lacorte, A. Gottesdiener, R. Bounds, S. Sharma, C. Folster, B. Henderson, S. O’Rahilly, E. Stoner, K. Gottesdiener, B. L. Panaro, R. D. Cone, K. Clement, I. S. Farooqi, and L. H. T. Van der Ploeg. 2017. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Molecular Metabolism 6(10):1321-1329. https://doi.org/10.1016/j.molmet.2017.06.015
- Rhythm Pharmaceuticals. 2017. Pipeline. Available at: http://www.rhythmtx.com/pipeline/product-pipeline (accessed March 14, 2018).
- Davies, M., T. R. Pieber, M. L. Hartoft-Nielsen, O. K. H. Hansen, S. Jabbour, and J. Rosenstock. 2017. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: A randomized clinical trial. JAMA 318(15):1460-1470. https://doi.org/10.1001/jama.2017.14752
- Drucker, D. J., J. F. Habener, and J. J. Holst. 2017. Discovery, characterization, and clinical development of the glucagon-like peptides. Journal of Clinical Investigation 127(12):4217-4227. Available at: https://www.jci.org/articles/view/97233 (accessed September 1, 2020).
- Neal, B., V. Perkovic, K. W. Mahaffey, D. de Zeeuw, G. Fulcher, N. Erondu, W. Shaw, G. Law, M. Desai, D. R. Matthews, and Canvas Program Collaborative Group. 2017. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine 377(7):644-657. https://doi.org/10.1056/NEJMoa1611925
- Hollander, P., H. E. Bays, J. Rosenstock, M. E. Frustaci, A. Fung, F. Vercruysse, and N. Erondu. 2017. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: A randomized clinical trial. Diabetes Care 40(5):632-639. https://doi.org/10.2337/dc16-2427
- Ziauddeen, H., S. R. Chamberlain, P. J. Nathan, A. Koch, K. Maltby, M. Bush, W. X. Tao, A. Napolitano, A. L. Skeggs, A. C. Brooke, L. Cheke, N. S. Clayton, I. Sadaf Farooqi, S. O’Rahilly, D. Waterworth, K. Song, L. Hosking, D. B. Richards, P. C. Fletcher, and E. T. Bullmore. 2013. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: A proof of mechanism study in binge-eating obese subjects. Molecular Psychiatry 18(12):1287-1293. https://doi.org/10.1038/mp.2012.154
- Morgan, R. A., K. R. Beck, M. Nixon, N. Z. M. Homer, A. A. Crawford, D. Melchers, R. Houtman, O. C. Meijer, A. Stomby, A. J. Anderson, R. Upreti, R. H. Stimson, T. Olsson, T. Michoel, A. Cohain, A. Ruusalepp, E. E. Schadt, J. L. M. Bjorkegren, R. Andrew, C. J. Kenyon, P. W. F. Hadoke, A. Odermatt, J. A. Keen, and B. R. Walker. 2017. Carbonyl reductase 1 catalyzes 20beta-reduction of glucocorticoids, modulating receptor activation and metabolic complications of obesity. Scientific Reports 7(1):10633. Available at: https://www.research.ed.ac.uk/portal/en/publications/carbonyl-reductase-1-catalyzes-20reduction-of-glucocorticoids-modulating-receptor-activation-and-metabolic-complications-of-obesity(a63331d0-ca0e-449a-8f5e-0a515dabbb60)/export.html (accessed September 1, 2020).
- US Food and Drug Administration. 2018. Obesity treatment devices. Available at: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ObesityDevices/default.htm (accessed March 5, 2018).
- Heshmati, H. M., Study of Gelesis100 on body weight in overweight and obese subjects with and without type 2 diabetes. 2014. National Library of Medicine: ClinicalTrials.gov.
- Lillis, J., J. G. Thomas, H. M. Niemeier, and R. R. Wing. 2017. Exploring process variables through which acceptance-based behavioral interventions may improve weight loss maintenance. Journal of Contextual Behavioral Science 6(4):398-403. https://doi.org/10.1016/j.jcbs.2017.07.005
- Martin, C. K., A. C. Miller, D. M. Thomas, C. M. Champagne, H. Han, and T. Church. 2015. Efficacy of SmartLoss, a smartphone-based weight loss intervention: Results from a randomized controlled trial. Obesity (Silver Spring) 23(5):935-942. https://doi.org/10.1002/oby.21063
- Martin, C. K., T. Nicklas, B. Gunturk, J. B. Correa, H. R. Allen, and C. Champagne. 2014. Measuring food intake with digital photography. Journal of Human Nutrition and Dietetics 27 Suppl 1:72-81. https://doi.org/10.1111/jhn.12014
- Schauer, D. P., H. S. Feigelson, C. Koebnick, B. Caan, S. Weinmann, A. C. Leonard, J. D. Powers, P. R. Yenumula, and D. E. Arterburn. 2017. Bariatric surgery and the risk of cancer in a large multisite cohort. Annals of Surgery. https://doi.org/10.1097/ SLA.0000000000002525