Cancer Prevention and Control: Then and Now
Controlling or reducing the risk for cancer was a principal goal of the National Cancer Act, signed by President Nixon in 1971. Long before the human genome was sequenced, and the etiology of many cancers was understood, Americans were determined to find a way to reduce the burden of cancer in the population. The act was passed only seven years after the first US Surgeon General’s report on the health consequences of cigarettes and smoking. As we approach the 50th year since the War on Cancer began, it is helpful to reflect on past and current cancer prevention and control. This paper focuses on 1) early detection and prevention of cancer and 2) cancer survivorship, areas highlighted in the recent Lancet Oncology Commission report on future cancer research priorities in the United States [1].
Early Detection and Prevention of Cancer
Cervical Cancer
In the early 1970s, the only established cancer screening test was the cervical cancer Papanicolaou (Pap) smear. During a woman’s annual pelvic examination, cervical material was spread on a glass slide, dropped in fixative, and sent for examination. Clinicians understood that cancers could be detected in this way, but they had little knowledge of cervical cancer’s natural history and its relationship to various risk factors (i.e., sexual behavior, human papillomavirus [HPV] infection, or smoking). Clinicians had only begun to routinely screen younger women who were sexually active. Cervical cancer was the number one cause of female cancer deaths worldwide, prematurely taking the lives of women in their thirties and forties. I recall taking care of young women who received a radical surgical procedure called pelvic exenteration— an attempt to eradicate advanced cervical cancer through removal of the cervix, uterus, ovaries, bladder, lymph nodes, and sometimes bowel. Radiation therapy was ineffective in preventing the return of the disease, and death by renal failure was common, due to obstruction of the drainage system from the kidneys. While radical hysterectomy is still performed today, the exenteration surgical procedure was abandoned due to its extreme morbidity and failure to prevent both local and distant metastatic disease.
Today, we understand that almost all cervical cancer results from infection with oncogenic serotypes of HPV that are sexually transmitted. Pap smears are still performed, but at less frequent intervals. Testing for high-risk HPV DNA can be conducted instead, or co-testing can be performed [2]. There are several highly effective vaccines that protect against HPV infection. Active campaigns are underway to immunize all pre-teenage girls and boys, although uptake has been lower than expected [3]. Immunization as a cancer prevention strategy has already shown benefit for hepatitis B virus and liver cancer [4]. In the United States, HPV immunization has taken on even greater importance because of an epidemic of other HPV-associated malignancies (oropharynx, anal), increasing the cancer prevention value of HPV immunization [5].
There are about 10,000 new cases of cervical cancer each year in the United States. In spite of highly effective tests for early detection, many women are diagnosed with advanced local or metastatic disease and have a five-year survival rate that is just over 60 percent [6]. Disparities in treatment and survival outcomes are substantial, especially for African American women, and much more needs to be done to improve prevention and early detection of cervical cancer in all women through immunization and screening.
Prostate Cancer
Other evidence-based cancer screening tests have been introduced over the past decades (e.g., mammography for breast cancer, and fecal occult blood tests, sigmoidoscopy, and colonoscopy for colorectal cancer) [7]. Some tests were adopted without sufficient evidence, for example, use of the prostate-specific antigen (PSA) blood test for prostate cancer screening. The PSA test was developed to detect metastatic recurrence in prostate cancer patients, but in the 1980s, the PSA test was rapidly adopted as a screening test without evidence that it reduced mortality from prostate cancer. This led to an explosion in the number of prostate cancers diagnosed each year, peaking in 1992 [8,9]. Ultimately, it was recognized that the PSA test identified a reservoir of indolent prostate cancers whose early detection and treatment did not decrease mortality. Prostate cancer screening guidelines have since been revised, and recommendations for PSA screening are much more limited to populations at increased risk [8,9]. This was one of the earliest examples of an “over diagnosis of cancer”, in which screening detects nonlethal, early cancers. Once an early cancer is detected, the person is likely to receive complex therapy that can have significant and long-term side effects, but the treatments do not alter the person’s lifespan.
Breast Cancer
Screening mammography can reduce mortality from breast cancer among women. However, implementation of regular mammography screening also led to a rapid expansion of cases of ductal carcinoma in situ—a noninvasive pre-cancer that was virtually nonexistent in the 1970s, but now accounts for about 60,000 new cases of breast cancer each year. As in the case of prostate cancer, many of these early breast abnormalities will not develop into lethal cancers. In order to maximize benefits and reduce the harms of screening, guideline developers are currently struggling to recommend and implement more rational, risk-based approaches to breast cancer screening, matching the woman’s risk for disease, based on age and other factors, with the appropriate screening interval and technology. Example strategies include implementing more frequent screening in those at high risk for breast cancer and less frequent screening in those at low risk, to minimize the likelihood of overdiagnosis and treatment of indolent disease [10].
Lung Cancer
Lung cancer is the primary cause of cancer mortality in both men and women. Recently, the National Cancer Institute completed a very large lung cancer screening trial using low-dose computerized tomographic (CT) chest imaging in high-risk individuals [11]. The trial was a success in terms of documenting a statistically significant mortality reduction associated with screening. However, there are significant challenges in implementing effective screening programs, including variable access to the technology and clinical expertise needed for screening [12,29]. Screening should also be coupled with interventions for tobacco cessation, which can greatly reduce the risk of lung cancer and other life-threatening conditions. Many individuals who are eligible for screening are also not receiving this test due to lack of insurance to pay for the screening and the post-screening medical evaluations that may be necessary. In addition, low-dose chest CT screening often identifies other incidental radiographic findings, some of which are serious and others that are benign, leading to further invasive procedures and unnecessary testing. Thus, low-dose chest CT lung cancer screening presents new ethical, social, and financial challenges, and also suffers from “over diagnosis” [13].
Genetic Testing for Hereditary Cancers
Cancer is now preventable for a segment of the population who have certain inherited cancer susceptibility genes [14]. There have been great advances in knowledge about familial cancer syndromes through initial genetic studies in families with multi-generational early onset cancers (e.g., breast, colon, ovary, uterus) and subsequent identification of the associated genes. More knowledge has also come from advances associated with the Human Genome Project and laboratory and commercial developments leading to the widespread availability of clinical testing. Genetic counseling and testing for cancer predisposition is now widely available, providing an opportunity for carriers of deleterious gene mutations to engage in more intensive screening, chemoprevention, or risk-reducing surgical procedures. Although the opportunity to prevent cancers in these families is at hand, access and uptake (care delivery) barriers still exist and highlight an important missed opportunity [15].
Cancer Survivorship
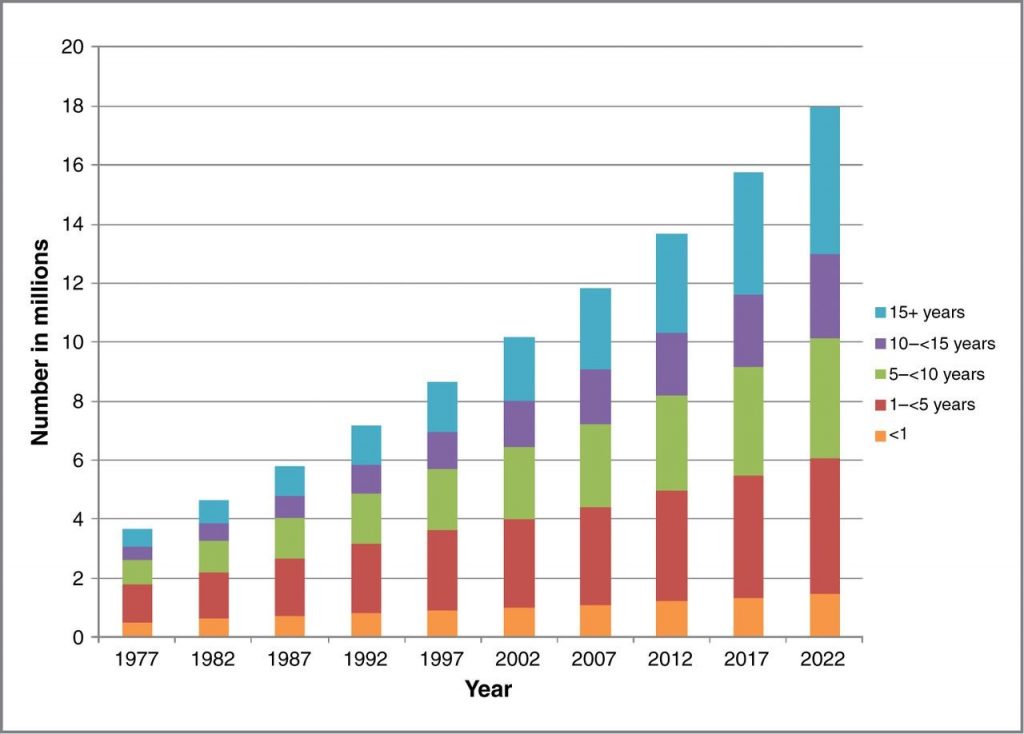
There are over 15 million cancer survivors today, with 18 million expected by 2022 [16] (Figure 1). When the National Cancer Act was signed, there were about 3 million cancer survivors. Great strides in early detection and more effective treatments due to better understanding of the biology of cancer have improved survival and patient outcomes, but these advances have not come without substantial personal and economic costs to patients and their families [17,18].
Figure 1 | Cancer Survivors in the United States
Estimated and projected number of cancer survivors in the United States from 1977 to 2022, by years since diagnosis.
SOURCE: De Moor, J. S., A. B. Mariotto, C. Parry, C. M. Alfano, L. Padgett, E. E. Kent, L. Forsythe, S. Scoppa, M. Hachey, and J. H. Rowland. 2013. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiology, Biomarkers & Prevention 22(4):561-570. Reprinted with permission.
Fifty years ago, most cancers were treated with extensive surgical resections. For example, the radical mastectomy for breast cancer was initiated by William Stewart Halsted in the late 19th century for control of tumors that often involved the entire breast and were adherent to the muscles of the chest wall. Removal of the entire breast, the pectoral muscles, and all of the lymph nodes under the arm was deemed essential for curing the cancer and preventing it from spreading elsewhere in the body [19]. However, there was no systematic study of this procedure until a randomized clinical trial in the early 1970s demonstrated that there was no survival benefit from the more extensive surgical procedure [20]. Subsequent clinical trials found that it was not necessary to remove the whole breast and that survival was just as good after removing the lump and radiating the remaining breast tissue [21]. Indeed, patients with small tumors in the breast have life expectancies that are similar to unaffected women their age, making it reasonable to avoid mutilating surgical procedures [22].
Many studies in a variety of solid tumors found that the propensity for cancers to metastasize and cause death was closely associated with the extent of the tumors at diagnosis (cancer staging). Additional research demonstrated that cancer cells from primary tumors often spread to distant organs (lung, bone, liver, brain) early in the course of tumor growth. These occult metastatic deposits would become apparent months to years after removal of the primary tumor. This spawned the clinical evaluation of adjuvant therapies—treatments given to control occult microscopic disease—when there was no evidence of cancer but high risk for recurrence. Adjuvant therapy is responsible for the high cure rate for many cancer patients and the growing number of survivors. Today, such therapies (chemotherapy, targeted therapy, endocrine therapy) are often given before the tumor is removed (neoadjuvant therapy). This order of treatment provides an opportunity to monitor treatment response in the primary tumor, whose complete disappearance is linked to the likelihood of control of any distant metastatic disease. Further, clinicians are able to biologically profile many cancers today, so specific genetic mutations or biological features allow oncologists to provide more specific, targeted treatments based on biology, not on how the tumor looks under the microscope. This can also help patients avoid unnecessary treatments for low-risk cancers [23].
In addition to increasing the number of adult cancer survivors, these advances have led to even more striking achievements in the treatment of children with cancer. As the War on Cancer began, average survival times for children with acute leukemia were only a few months. Serial and coordinated clinical treatment trials have resulted in long-term survival for more than 90 percent of children diagnosed with leukemia today. Other types of childhood cancers (e.g., brain tumors, lymphoma, sarcoma) have also experienced significantly improved outcomes. This is a major triumph resulting from basic and clinical cancer research during the past 50 years. However, this achievement is blemished by the shortened lifespan of many childhood cancer survivors, largely resulting from other chronic treatment-related health conditions that lead to ongoing morbidity and premature death [24]. The challenge for pediatric cancer specialists today is how to deescalate very intensive curative treatments and to decrease and eliminate the well-described adverse outcomes.
Demographic changes associated with an aging population will lead to an even larger number of adult cancer survivors in the years ahead. The cancer screening advances described earlier will increase early detection of the common age-related cancers (breast, prostate, colorectal, and lung), and that will enable less aggressive treatment. Clinical treatment trials supported by the government, foundations, and industry are applying preclinical discoveries to yield successful therapeutic strategies, with accelerated approval of many new treatments in recent years that now lead to high cure rates in many hematologic malignancies and solid tumors. Many new therapies are targeted to specific genetic mutations, such as imatinib (commercially known as Gleevec) to target the BCR-ABL1mutation associated with chronic myelogenous leukemia (CML). Gleevec has transformed CML from a uniformly fatal malignancy into a disease that can be successfully treated with a chronic oral medication and allows patients to avoid high-dose chemotherapy and stem cell rescue [25]. Understanding the molecular and genomic features of tumors has improved the effectiveness of treatments and has decreased use of toxic treatments in subpopulations of patients.
One of our biggest challenges is how to provide high-quality care for the growing number of cancer patients and survivors [26]. Multiple oncology specialists participate in a patient’s initial cancer treatment, leading to fragmentation of care. After completion of primary treatment, there is limited coordination of care between oncology specialists and primary care clinicians. Given the extended survival for most cancer patients, as well as comorbidities acquired from treatment and aging, cancer survivors need better strategies for long-term follow-up. This includes management of post-treatment symptoms, disease prevention, and health promotion [27]. While surveillance for cancer recurrence is a priority, cancer survivors are also at substantially increased risk for new cancers [28]. About 15 to 20 percent of incident cancers each year occur in individuals with a past history of cancer, and this is especially an issue among elderly patients. New models of care are required to optimize the coordination of care for cancer patients during treatment and as they transition back to primary care after the completion of treatment [18].
Conclusion
We have come a long way in understanding the etiologies, pathogeneses, and natural histories of many cancers. This has led to many effective interventions for cancer prevention, screening, diagnosis, and treatment. However, the widespread public health messages about the value of “early diagnosis,” championed by many cancer advocacy organizations and national guidelines, have also put us at risk of doing more harm than good when it comes to cancer screening. It is very hard to modify the extensive communications to both the public and clinicians regarding early detection. The ability to do less frequent and more targeted screening to optimize the risk/benefit tradeoffs is the challenge we face currently. While implementing more strategic prevention, screening, and early detection efforts, we must not overlook important disparities in the receipt of evidence-based care that can reduce the burden of cancer in high-risk and vulnerable populations.
Continuing growth in the numbers of cancer survivors reflects the tremendous success of the War on Cancer, but for many cancer patients, the effects of treatment are not over when treatment ends. Investments in basic and clinical/translational research, clinical trials, and a surge in new cancer treatments are propelling improvements in survival. Many patients are living cancer-free. Others engage in chronic therapy and their disease is controlled. Immunotherapy is the current “hot area” of treatment being employed or tested across many cancer sites; however, we know little about the potential late effects of these new therapies. While systematic survivorship research has advanced our knowledge about the long-term and late effects of past cancer treatments, we still have much to learn about these new therapeutic approaches. In addition, the health care system must be prepared to address the physical, psychosocial, and financial toxicities that face the existing population of cancer survivors and their families. Thus, the future of cancer prevention and control research is full of many opportunities and challenges.
Join the conversation!
![]() Tweet this! Despite significant progress in detecting and controlling #cancer, disparities persist in high-risk and vulnerable populations and must be addressed: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
Tweet this! Despite significant progress in detecting and controlling #cancer, disparities persist in high-risk and vulnerable populations and must be addressed: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
![]() Tweet this! .@theNAMedicine member Patricia Ganz looks back at the progress of #cancer prevention and control, 50 years after the War on Cancer began: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
Tweet this! .@theNAMedicine member Patricia Ganz looks back at the progress of #cancer prevention and control, 50 years after the War on Cancer began: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
![]() Tweet this! There are over 15 million #cancer survivors today, compared to 3 million in 1971. Providing high-quality care to these individuals is challenging, but critical: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
Tweet this! There are over 15 million #cancer survivors today, compared to 3 million in 1971. Providing high-quality care to these individuals is challenging, but critical: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
![]() Tweet this! Targeted, specific, and genetically-based tests to screen for #cancers are becoming more of a reality every day, and will reduce the number of false positives from broader cancer screening: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
Tweet this! Targeted, specific, and genetically-based tests to screen for #cancers are becoming more of a reality every day, and will reduce the number of false positives from broader cancer screening: https://doi.org/10.31478/201810f #NAMPerspectives #NAMMtg
Download these graphics and share them on social media!
References
- Jaffee, E. M., C. V. Dang, D. B. Agus, B. M. Alexander, K. C. Anderson, A. Ashworth, A. D. Barker, R. Bastani, S. Bhatia, J. A. Bluestone, O. Brawley, A. J. Butte, D. G. Coit, N. E. Davidson, M. Davis, R. A. DePinho, R. B. Diasio, G. Draetta, A. L. Frazier, A. Futreal, S. S. Gambhir, P. A. Ganz, L. Garraway, S. Gerson, S. Gupta, J. Heath, R. I. Hoff man, C. Hudis, C. Hughes-Halbert, R. Ibrahim, H. Jadvar, B. Kavanagh, R. Kittles, Q.-T. Le, S. M. Lippman, D. Mankoff , E. R. Mardis, D. K. Mayer, K. McMasters, N. J. Meropol, B. Mitchell, P. Naredi, D. Ornish, T. M. Pawlik, J. Peppercorn, M. G. Pomper, D. Raghavan, C. Ritchie, S. W. Schwarz, R. Sullivan, R. Wahl, J. D. Wolchok, S. L. Wong, A. Yung. 2017. Future cancer research priorities in the USA: A Lancet Oncology Commission. The Lancet Oncology 18(11):e653-e706. https://doi.org/10.1016/S1470-2045(17)30698-8
- US Preventive Services Task Force. 2018. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 320(7):674-686. https://doi.org/10.1001/jama.2018.10897
- Strengthening the effectiveness of national, state, and local efforts to improve HPV vaccination coverage in the United States: Recommendations from the National Vaccine Advisory Committee. 2018. Public Health Reports: 0033354918793629. Washington, DC. https://doi.org/10.1177/0033354918793629
- Chen, C. J. 2018. Global elimination of viral hepatitis and hepatocellular carcinoma: Opportunities and challenges. Gut 67(4):595-598. https://doi.org/10.1136/gutjnl-2017-315407
- Van Dyne, E. A., S. J. Henley, M. Saraiya, C. C. Thomas, L. E. Markowitz, and V. B. Benard. 2018. Trends in human papillomavirus-associated cancers—United States, 1999–2015. Morbidity and Mortality Weekly Report 67:918-924. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6733a2.htm (accessed September 2, 2020).
- Benard, V. B., M. Watson, M. Saraiya, R. Harewood, J. S. Townsend, A. M. Stroup, H. K. Weir, and C. Allemani. 2017. Cervical cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer 123(S24):5119-5137. https://doi.org/10.1002/cncr.30906
- Smith, R. A., K S. Andrews, D. Brooks, S. A. Fedewa, D. Manassaram-Baptiste, D. Saslow, O. W. Brawley, and R. C. Wender. 2018. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians 68(4):297-316. https://doi.org/10.3322/caac.21446
- Helgstrand, J. T., M. A. Røder, N. Klemann, B. G. Toft, D. Y. Lichtensztajn, J. D. Brooks, K. Brasso, B. Vainer, and P. Iversen. 2018. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer—A population-based analysis of 2 national cohorts. Cancer 124(14):2931-2938. https://doi.org/10.1002/cncr.31384
- Brawley, O. W. 2018. Prostate cancer screening: And the pendulum swings. Cancer 124(14):2890-2892. https://doi.org/10.1002/cncr.31380
- Esserman, L. J., I. M. Thompson, B. Reid, P. Nelson, D. F. Ransohoff , H. G. Welch, S. Hwang, D. A. Berry, K. W. Kinzler, W. C. Black, M. Bissell, H. Parnes, and S. Srivastava. 2014. Addressing overdiagnosis and overtreatment in cancer: A prescription for change. The Lancet Oncology 15(6):e234-e242. https://doi.org/10.1016/S1470-2045(13)70598-9
- National Lung Screening Trial Research Team. 2013. Results of initial low-dose computed tomographic screening for lung cancer. The New England Journal of Medicine 368(21):1980-1991. https://doi.org/10.1056/NEJMoa1209120
- Deffebach, M. E., and L. Humphrey. 2015. Lung cancer screening. Surgical Clinics of North America 95(5):967-978. https://doi.org/10.1016/j.suc.2015.05.006
- Ebell, M. H., and K. W. Lin. 2018. Accounting for the harms of lung cancer screening. JAMA Internal Medicine. https://doi.org/10.1001/jamainternmed.2018.3061
- Li, F. P., and A. S. Whittemore. 1999. Genetically tailored preventive strategies: An effective plan for the twenty-first century? Cancer Epidemiology, Biomarkers & Prevention 8(8):649-658. Available at: https://cebp.aacrjournals.org/content/8/8/649 (accessed September 2, 2020).
- Childers, C. P., K. K. Childers, M. Maggard-Gibbons, and J. Macinko. 2017. National estimates of genetic testing in women with a history of breast or ovarian cancer. Journal of Clinical Oncology 35(34):3800-3806. https://doi.org/10.1200/jco.2017.73.6314
- De Moor, J. S., A. B. Mariotto, C. Parry, C. M. Alfano, L. Padgett, E. E. Kent, L. Forsythe, S. Scoppa, M. Hachey, and J. H. Rowland. 2013. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiology, Biomarkers & Prevention 22(4):561-570. https://doi.org/10.1158/1055-9965.EPI-12-1356
-
Institute of Medicine and National Research Council. 2006. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press. https://doi.org/10.17226/11468
- National Academies of Sciences, Engineering, and Medicine. 2018. Long-Term Survivorship Care After Cancer Treatment: Proceedings of a Workshop. Washington, DC: The National Academies Press. https://doi.org/10.17226/25043
- Fisher, B., J.-H. Jeong, S. Anderson, J. Bryant, E. R. Fisher, and N. Wolmark. 2002. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. The New England Journal of Medicine 347(8):567-575. https://doi.org/10.1056/NEJMoa020128
- Bland, C. S. 1981. The Halsted mastectomy: Present illness and past history. Western Journal of Medicine 134(6):549-555. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1272864/ (accessed September 2, 2020).
- Fisher, B., M. Bauer, R. Margolese, R. Poisson, Y. Pilch, C. Redmond, E. R. Fisher, N. Wolmark, M. Deutsch, and E. Montague. 1985. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. The New England Journal of Medicine 312(11):665-673. https://doi.org/10.1056/nejm198503143121101
- DeSantis, C. E., S. A. Fedewa, A. Goding Sauer, J. L. Kramer, R. A. Smith, and A. Jemal. 2016. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians 66(1):31-42. Available at: https://www.unboundmedicine.com/medline/citation/26513636/Breast_cancer_statistics_2015:_Convergence_of_incidence_rates_between_black_and_white_women_ (accessed September 2, 2020).
- Sparano, J. A., R. J. Gray, D. F. Makower, K. I. Pritchard, K. S. Albain, D. F. Hayes, C. E. Geyer, Jr., E. C. Dees, M. P. Goetz, J. A. Olson, Jr., T. Lively, S. S. Badve, T. J. Saphner, L. I. Wagner, T. J. Whelan, M. J. Ellis, S. Paik, W. C. Wood, P. M. Ravdin, M. M. Keane, H. L. Gomez Moreno, P. S. Reddy, T. F. Goggins, I. A. Mayer, A. M. Brufsky, D. L. Toppmeyer, V. G. Kaklamani, J. L. Berenberg, J. Abrams, and G. W. Sledge, Jr. 2018. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. The New England Journal of Medicine 379(2):111-121. https://doi.org/10.1056/NEJMoa1804710
- Armstrong, G. T., T. Kawashima, W. Leisenring, K. Stratton, M. Stovall, M. M. Hudson, C. A. Sklar, L. L. Robison, and K. C. Oeffinger. 2014. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. Journal of Clinical Oncology 32(12):1218-1227. https://doi.org/10.1200/JCO.2013.51.1055
- Soverini, S., C. De Benedittis, M. Mancini, and G. Martinelli. Best practices in chronic myeloid leukemia monitoring and management. The Oncologist 21(5):626-633. https://doi.org/10.1634/theoncologist.2015-0337
- Institute of Medicine. 2013. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press. https://doi.org/10.17226/18359
- Ganz, P. A. 2011. The “three Ps” of cancer survivorship care. BMC 9(1):14. https://doi.org/10.1186/1741-7015-9-14
- Murphy, C. C., D. E. Gerber, and S. L. Pruitt. 2018. Prevalence of prior cancer among persons newly diagnosed with cancer: An initial report from the Surveillance, Epidemiology, and End Results program. JAMA Oncology 4(6):832-836. https://doi.org/10.1001/jamaoncol.2017.3605
- National Academies of Sciences, Engineering, and Medicine. 2017. Implementation of lung cancer screening: Proceedings of a workshop. Washington, DC: The National Academies Press. https://doi.org/10.172216/23680